19 Sept 2023
Victoria Colgate and Richard Newton discuss the concerns vets have over the potential gateways for global pathogen spread in this species.

Image © hedgehog94 / Adobe Stock
Defined as diseases that are not usually present in a given country (Spence et al, 2019; Spence et al, 2022), incursions of exotic equine diseases are historically seemingly rare in the UK. However, the changing world we live in and the potentially profound impacts from disease entry, mean they must not be overlooked.
Indeed, the international connectivity of the equine industry, combined with globalisation of trade and climate change increasing the suitability of vector habitats, opens many potential gateways for pathogen spread.
Juxtaposed to this is a generally poor level of knowledge and awareness among horse owners about the potential exotic diseases that could arrive in the UK.
We must strive to communicate this risk, without engendering panic, to ensure the early detection of future incursions; a vital step towards minimising impact through timely implementation of control measures.
Under UK animal health legislation, most exotic diseases that could spread to the UK are classified as “notifiable” and a large number, both in horses and other species, are also vector-borne (Whitlock et al, 2023).
To some extent, it is this mode of transmission, via competent vectors, that means exotic diseases are an increasing threat as they spread ever-closer to our shores.
Climate change, most notably global warming and increased incidence of extreme weather events, is allowing vectors and any pathogens carried by them to expand their spatial distribution into previously uninhabited areas (D’Amore et al, 2022).
This means that, whether an exotic pathogen enters within an infected vector or by another route, the presence of a suitable habitat and vector species within the receiving country will help support a successful transmission cycle following incursion. Hence, isolated entry of exotic pathogens has the potential to escalate into extended circulation, and potentially subsequent establishment of endemicity.
It may seem excessive to focus on these “what-ifs”, but recent international equine infectious disease occurrence provides a cautionary tale, and highlights how tangible a threat this really is.
African horse sickness (AHS) is a highly infectious disease of equids, spread by midges of the Culicoides species and with a mortality rate approaching 90% (Lo Iacono et al, 2013) in a naive population. Disease occurrence is associated with instigation of international trade bans and extensive disease control costs, which on top of the horse welfare and associated socio-economic impacts, means that incursion has potentially ruinous consequences (Assefa et al, 2022).
Although normally restricted to sub-Saharan Africa, AHS virus (AHSV) transmission has resulted in occasional sustained outbreaks outside of this region, as demonstrated by its incursion into Spain in 1987. A group of sub-clinically AHSV-infected zebra was imported and held in an outdoor quarantine area near Madrid (Chapman et al, 2018a), leading to infection of competent vectors and, due to delayed detection, a spreading epidemic that affected Spain, Portugal and Morocco and resulted in the death of approximately 1,400 equids across a four-year period (Chapman et al, 2018b).
This highlights the potential for rapid spread into new areas, the danger of disease introduction with the importation of animals, and how delayed identification of an exotic disease incursion can lead to preventable escalation.
More recently, AHS emerged in Thailand in 2020, once again in association with the importation of zebra from Africa (Nelson et al, 2022). As AHS has never entered the UK, these threats may still seem far away, but the UK’s experiences with bluetongue virus (BTV) tell a different story.
BTV and AHSV are closely related orbiviruses that share the same vector species. Prior to 2006, BTV had not occurred in northern Europe, believed to be due to the lack of a competent midge vector species in this region (Robin, 2019). However, the 2006-08 BTV-8 European outbreak led to a rapid spread of disease, affecting large numbers of farms, including in the UK.
Eradicated with the assistance of vaccination, the UK remains at continued risk of BTV re-introduction – particularly from Europe, where it remains endemic. Most importantly, however, the BTV-8 outbreak showed that northern European midge species were competent vectors for orbiviruses such as BTV and AHSV, most likely assisted by climate change altering vector dynamics (Robin, 2019).
Historically, AHS incursion into the UK was thought of as very low-risk, which it still is, but the BTV-8 outbreak illustrated that AHS entry must now be regarded as a possibility due to their sharing of vector species (Robin, 2019).
Similarly, the 2011-12 Schmallenberg outbreak affecting ruminants highlighted how quickly vector-borne diseases can spread into new areas, with highly impactful consequences (de Vos et al, 2021).
It is important to remember, however, that not all exotic diseases are vector-borne. Contagious equine metritis (CEM) is a venereal disease of equids that causes purulent endometrial and vaginal discharge and associated infertility. Following its emergence and first recognition in Newmarket in 1977, the disease was eradicated from the affected UK population through the use of pre-breeding diagnostic screening and control measures that were developed into an industry-adopted code of practice.
However, being endemic in many parts of Europe, CEM continues to be periodically confirmed in the UK linked to the importation of subclinically infected horses (Figure 1).
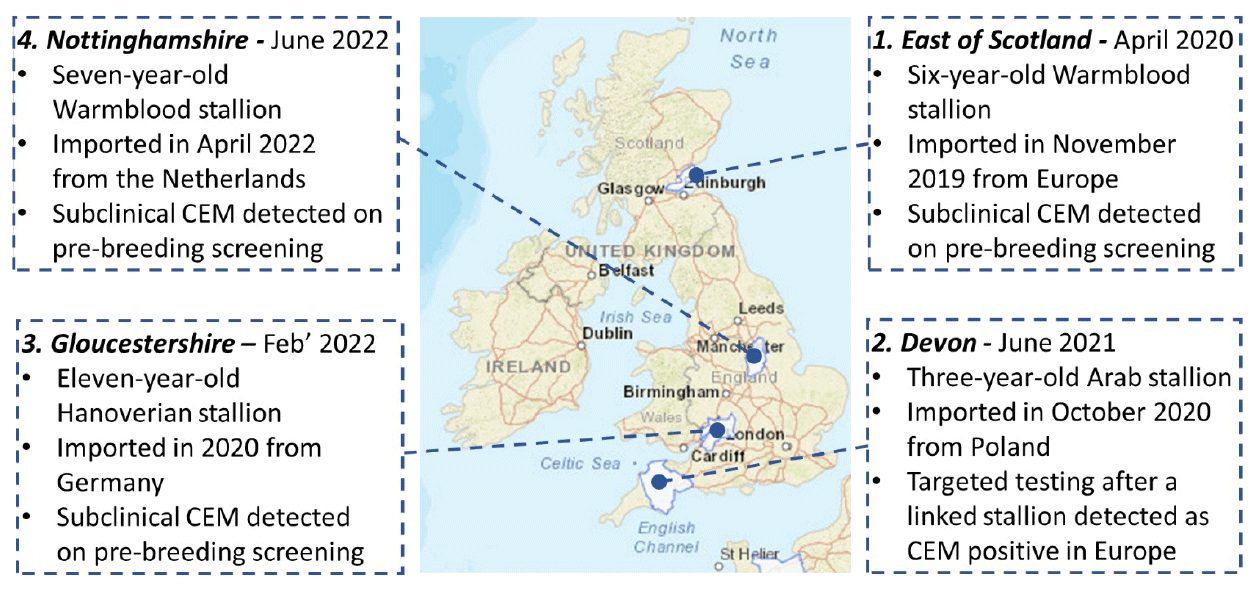
Although voluntary pre-breeding testing requirements (Horserace Betting Levy Board International Codes of Practice; HBLB) are effective in identifying these cases and facilitate instigating of control measures to ensure rapid eradication, the repeat introductions highlight the potential for pathogen spread with the movement of equids.
Of particular note among exotic equine diseases that may become established in the UK is west Nile virus (WNV). WNV is a vector-borne flavivirus that was first identified in Uganda in 1937 and, since the late 1990s, has been re-emerging into southern European countries (Cavalleri et al, 2022) and the Mediterranean basin (Lecollinet et al, 2020).
WNV has a mosquito-bird transmission cycle, but humans and equids are also susceptible to infection. However, they are dead-end hosts that do not act as a source of infection for onward transmission.
No indigenously acquired autochthonous WNV infection has so far been detected in the UK (Folly et al, 2020), but its past explosive, and more recent steady, further expansion demonstrates how flaviviruses can emerge into new geographic areas with an often unpredictable pattern (Cavalleri et al, 2022). In 1999, the now-endemic WNV was first introduced into New York City, from where it rapidly spread across the US, causing more than 30,000 human and 24,000 recorded cases in equids across a 10-year-period (Lecollinet et al, 2020). This demonstrates the ability of an exotic pathogen to establish in naive populations, but in the same timeframe, Europe continued to experience regular seasonal outbreaks that were more limited in time and space (Lecollinet et al, 2020).
However, in 2018, Europe recorded unprecedented levels of WNV transmission, as both the number of reports and the geographical range of the virus increased (Browne and Medlock, 2019).
Notably, the virus spread further northwards, so that WNV was detected for the first time in birds and horses in Germany in 2018 (Ganzenberg et al, 2022), and in humans and birds (but not horses) in the Netherlands in 2020 (de Vos et al, 2021). This unusually heightened WNV activity in 2018 was associated with a 1.3°C rise in the highest recorded summer temperatures in Europe (Browne and Medlock, 2019), illustrating how climate change is potentially expanding vector, and in turn disease, territories.

Although autochthonous WNV infection has not yet been detected in the UK, suitable mosquito vector and avian amplifying host species are present here, and the recent expansion of WNV serves as a warning that it may be a matter of when, rather than if, it arrives (Ivens, 2016). This prediction is substantiated by the fact that the Usutu virus, which has related ecology and co-circulates with WNV in Europe (Cavalleri et al, 2022), has been detected in wild birds in the UK since 2020 (Lawson et al, 2022).
Additionally, so far, two WNV positive cases have been detected in horses in the UK, although in both instances, infection was acquired while travelling through Europe and, as dead-end hosts, no risk of further transmission to other susceptible horses, humans or mosquitoes existed.
The first case of equine WNV encephalomyelitis in the UK occurred in October 2013 and involved a horse that had recently arrived after travelling by road through mainland Europe, having originated from Cyprus (Gonzalez-Medina et al, 2017).
The second case (Figure 2), confirmed in November 2022, involved a horse that started to display neurological signs on the return journey to the UK after having been competing for several weeks in Southern Spain.
These cases act as an important reminder that while WNV is not currently circulating in the UK, it should be considered as a differential in the neurological horse, especially if it has a recent history of travel. Accordingly, it would be additionally prudent to recommend vaccination against WNV for all horses travelling to endemic areas, including Europe, during vector season (April-November for the northern hemisphere).
Another non-endemic disease of potential interest is equine piroplasmosis (EP). EP is the result of infection with one or both of the intracellular protozoal parasites Babesia caballi and Theileria equi (Coultous et al, 2023). It can have significant impacts on reproductive capacity and athletic performance within equine populations, as well as having the ability to create a long-term subclinical carrier state.
Although EP is not currently considered to be endemic in the UK, it is widespread throughout Europe and with no obligation to report cases (Coultous et al, 2023) or carry out pre-import screening for infection, the risk of entry into the UK cannot be considered negligible.
Indeed, a qualitative risk assessment using the World Organisation for Animal Health framework identified that entry of EP into the UK via the importation of horses with acute disease or infected ticks or blood was considered low risk, but entry through the importation of subclinical carrier animals was a medium risk (Coultous et al, 2023).
The same risk assessment further highlighted that EP positive animals are able to enter, and are probably present, in the UK (Coultous et al, 2023).
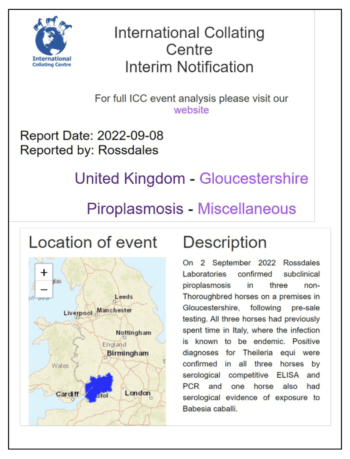
This is corroborated by the fact that in September 2022 the International Collating Centre reported three EP positive cases on a premises in Gloucestershire, all of which had recently spent time in Italy (Figure 3).
It was previously believed that EP had not established in the UK due to the absence of a suitable tick vector, but it is now known that capable species are present, albeit with a restricted UK distribution (Coultous et al, 2023).
Arguably, this calls for heightened surveillance and raised awareness of EP as an emerging pathogen that could increasingly been seen in, and impact, the UK equine industry.
Other exotic diseases that have on occasion entered the UK include equine infectious anaemia (EIA) and equine viral arteritis (EVA).
EIA is a trans-boundary disease with an almost worldwide distribution (Machado et al, 2021) which can be mechanically transmitted through a variety of vectors, as well as iatrogenically.
It has the ability to create a lifelong infection with persistence in leukocytes.
It is notable that EIA was historically endemic in Romania and this led to endemicity in Italy, following insufficient control in the face of infected horses entering from Romania (anonymous, 2011). Although Defra regards the risk of a horse travelling to an EIA infected region for a short period, becoming infected and leading to onward diseases transmission on its return to the UK, as very low (anonymous, 2011), it is a potential route of entry that should not be underestimated. Equine arteritis virus (EAV), the cause of EVA, can be spread by both venereal and respiratory routes, with a proportion of recovered stallions able to shed the virus in their semen for prolonged periods.
In the UK, EVA is a notifiable disease in stallions under the EVA Order (1995) and is not believed to be present in the country.
However, EVA remains endemic in some regions of Europe, providing a potential route of entry for EAV – particularly with the unrestricted importation of subclinically infected horses. In particular, the importation into the UK of EAV-positive, semen-shedding carrier stallions from Europe, either initially as competition horses or primarily to be used for breeding purposes, increases the risk of EVA entry and spread.
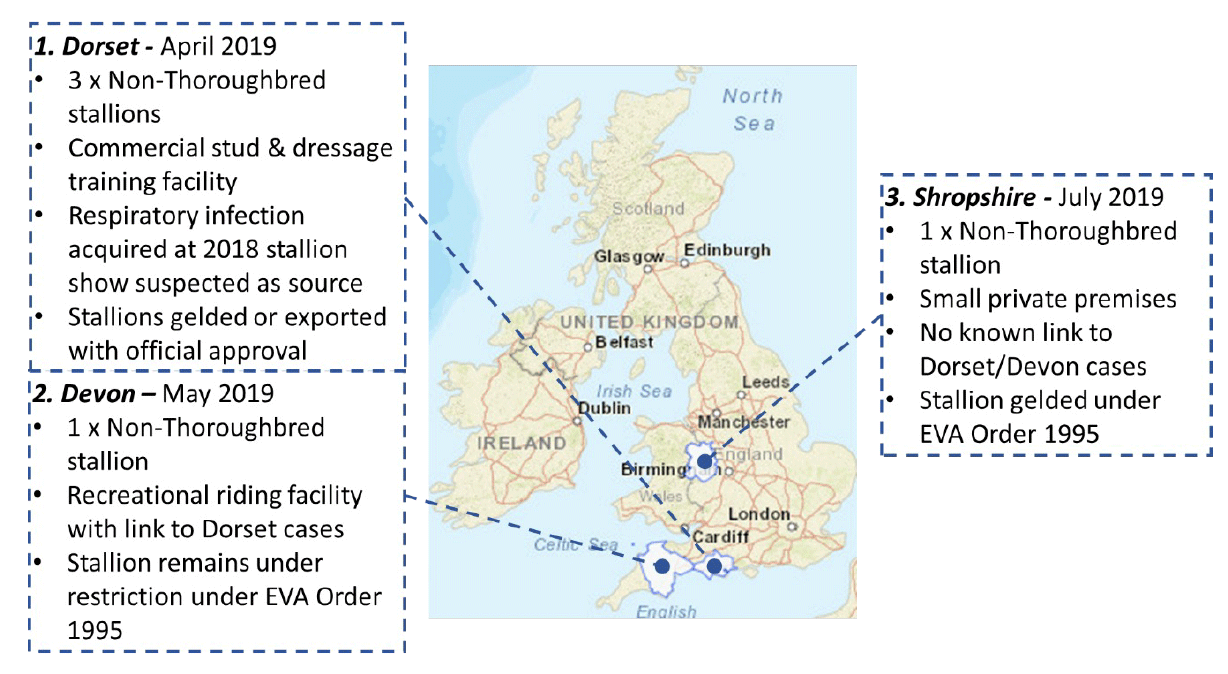
Further pathogen dissemination is currently controlled via voluntary pre-breeding screening regulations as outlined in the HBLB Codes of Practice, but this does not prevent the occurrence of isolated cases from time to time in the UK – the last of which was in 2019 (Figure 4).
While the UK currently remains separated from some of the exotic diseases that affect equids in mainland Europe and the rest of the world, increasing globalisation makes countries more inter-connected than ever before, and climate change is acting to expand the geographical distribution of many vector-borne diseases.
As the dynamics of equine infectious diseases begin to shift, our perceptions about them must update accordingly; they move up the list of our differential diagnoses, and we must strive to educate horse owners and colleagues to ensure any potential exotic disease incursion is not overlooked.
Only with proper disease preparedness and early detection can we hope to effectively control onward transmission and its potentially far-reaching consequences.