24 Apr 2017
Cara Hallowell-Evans and Gayle Hallowell discuss best practice methods of performing a worming egg count and how to interpret the results.

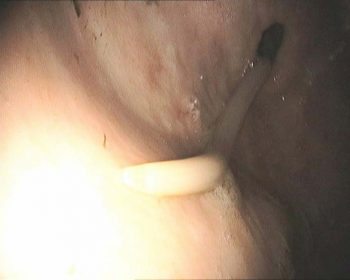
Figure 3. Image of an adult ascarid (Parascaris equorum) in the proximal part of the duodenum of a three-year-old warmblood mare under gastroscopy for evaluation of weight loss.
Intestinal parasites are ubiquitous in grazing horses and although most infections do not result in overt clinical disease under good management, the increasing prevalence of anthelmintic resistance presents a potential clinical challenge.
Intensive, traditional anthelmintic protocols initiated in the 1960s to control, at that time, highly prevalent and pathogenic Strongylus vulgaris infections are no longer recommended due to development of anthelmintic resistance in cyathostomin and Parascaris species. Anthelmintic resistance to multiple drug classes is widespread in these nematodes and, with a lack of novel drug development in the foreseeable future, the aim is to conserve the tools at our disposal.
As such, targeted regimes are being implemented in adult cohorts (for horses more than three years of age). To control and prevent clinical disease, faecal egg count (FEC) testing must be performed and needs to reflect parasite ecology, epidemiology and seasonality.
Everyone thinks performing a FEC is easy. However, to achieve accurate results it is essential to optimise diagnostics from sample collection to testing. Variable dispersion of eggs within the faecal pat necessitates collection of multiple samples from various areas of the faecal pile – five sampling sites or whole pellets is considered sufficient (Nielsen et al, 2010; Lester et al, 2012).
Due to the temperature-dependent nature of egg development, it is important samples are less than four hours old when collected, the air content of the bag needs to be minimised by compression prior to sealing to limit larval hatching and should be stored at 40°C (Nielsen et al, 2010). Testing should be undertaken as soon as possible after sampling – do not put samples in the post on Friday. These measures aim to preserve the egg content of the faeces by delaying larval development and hatching, which can result in a falsely reduced FEC, or worse, false-negative results.
Prior to sampling, thorough maceration and mixing of the sample should be performed to distribute eggs as evenly as possible (Lester et al, 2012). Various FEC flotation methods are available with a wide range of strongyle-type egg detection sensitivities (Figure 1) – these techniques were evaluated by Lester and Matthews (2014).
Ideally, testing should be performed using the highest sensitivity technique possible following the manufacturer or best practice methodology as, when egg detection limits are high, the test is not sensitive enough to detect changes in egg count, which is particularly important when undertaking sequential testing for drug efficacy evaluation (Lester and Matthews, 2014). The diagnostic test used, in combination with individual yard history, will influence the treatment threshold used for adult cohorts under targeted treatment protocols.
Due to natural variation in the egg output of individual horses, it is recommended FECs are used to evaluate a herd, with as many individuals as possible being tested (Denwood et al, 2012; Vidyashankar et al, 2012; American Association of Equine Practitioners [AAEP], 2013). The amount and variation of egg shedding in the herd can then be used to indicate the success of control measures (Relf et al, 2013).
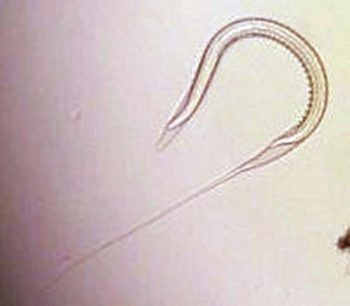
In well-managed adult (more than three years old) populations, strongyle-type egg distribution shows a negative binomial pattern with overdispersion of counts within the herd. The general rule, under good control conditions, is 20% of the herd contributes more than 80% of total egg output (“80:20 rule”), with the majority showing low or negative counts. With effective pasture and anthelmintic control conditions, the proportion of high shedders is often well below 20% (Relf et al, 2013).
For adult horses managed with targeted anthelmintic regimes, this principle is used to reduce shedding during periods of potentially high transmission (April to September) when climatic conditions will facilitate efficient development of parasitic eggs to infective third stage larvae (Figure 2). Testing within this regime is conducted at 8 to 12-week intervals, with an adulticidal anthelmintic administered only to high shedders, such as those excreting greater than 200 eggs per gram (EPG). This will reduce egg output and the infective pasture larval burden.
Several studies suggest individual horses with high FECs remain high throughout current and subsequent grazing seasons, although this finding is not consistent between all studies (Neilsen et al, 2006; Becher et al, 2010; Wood et al, 2012).
In youngstock, due to an increased risk of clinical disease and complication of ascarid burdens (Figure 3), targeted regimes are not advocated. Evaluation of pasture management strategies should be used with care as, within this age, cohort strongyle egg output does not adhere to the 80:20 rule (Relf et al, 2013). An added complication is the developing immunity to Parascaris infection, which can result in a declining or negative egg count even in the presence of a potentially pathogenic adult burden (Nielsen et al, 2015).
However, due to increasing reports of multidrug resistance in Parascaris species, FEC is a useful tool for determination of appropriate treatment regime and anthelmintic efficacy.
It is important to note egg counts do not give a quantitative indication of the number of adult or prepatent parasite stages present in the horse. This is especially important when considering prepatent stages may constitute up to 90% of the total parasite burden (Dowdall et al, 2002). FEC does provide an indication of the level of egg shedding and potential pasture contamination of an individual at a specific testing time point. This is of key importance when considering prevention of larval cyathostominosis and the limitations of FEC as a diagnostic tool for clinical disease.
Cyathostomins are considered the most important equine parasite due to their high prevalence and potential pathogenicity (Love et al, 1999). When management is insufficient, large numbers of encysted immature stages may accumulate over a period of years and emerge en-mass from the intestinal mucosa – resulting in severe clinical disease (Giles et al, 1985; Love et al, 1999). The trigger factor for this reactivation and emergence is unknown.
Signs of disease include rapid and severe weight loss (93% cases), diarrhoea (86%) and protein-losing enteropathy (hypoalbuminaemia in 71%; Mair et al, 2002). Full immunity does not develop. Although disease is most common in horses under six years old seen in late winter or early spring, it can be seen in any season or age (Abbott, 1998; Love et al, 1999). Mortality occurs in approximately 50% of cases.
Differentiation of larval cyathostominosis from other causes of diarrhoea can be extremely challenging. As the aetiology of this disease stems from prepatent stages, FECs of clinical cases are often negative. Immature worms may be seen in the faecal material or on a glove following rectal examination, but is not a reliable sign.
History may provide indicators of cyathostome-related disease, including unknown de-worming history in the preceding 6 to 12 months. Moxidectin usage is indicated in autumn to reduce the number of encysted stages and thus reduce disease risk. A positive association has been found between development of disease within a few weeks of ivermectin administration.
Faecal egg count reduction tests (FECRT) should be performed regularly in herds following both interval and targeted plans. It is important to note each parasite species should be tested separately for drug efficacy.
FECRT can also be implemented where there is suspicion of drug failure or reduced efficacy due to an increased level of clinical or subclinical suspected cases. Specific guidelines for interpretation of FECRT reduction percentages in horses are under development, as no universally agreed cut-off values for efficacy classification exist for horses.
FECRT involves FEC being undertaken prior to treatment and repeated two weeks later, with a calculation of relative change in EPG counts. Only horses recording an egg count greater than 50EPG should be included and, if less sensitive methodologies are used for FEC evaluation, it may be better to only include horses with counts more than 100EPG.
Due to overdispersion of egg shedding, only a small number of horses in a herd are likely to be eligible for inclusion, so testing as many of the herd prior to treatment is essential to ensure a minimum of six individuals are included in the FECRT. Those showing the highest pre-treatment egg counts should be used to undertake FECRT and the test should be performed at least 8 weeks after the last anthelmintic administration (or 12 weeks if moxidectin was used; AAEP, 2013).
As sensitive a test as possible should be used, preferably one with a detection limit of more than 25EPG (including modified McMaster FEC, modified Wisconsin, FECPAK, FLOTAC and centrifugal flotation techniques; AAEP, 2013; Lester and Matthews, 2014). For pre-treatment and post-treatment egg counts to be comparable, the same test and test sensitivity must be used, so samples should be sent to the same laboratory. The following is an accepted protocol for testing:
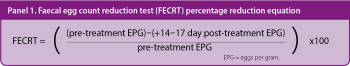
The percentage reduction in egg count is then compared to currently proposed cut-off values (Vidyashankar et al, 2007; Kaplan and Neilson, 2010). Reductions of more than 90% for benzimidazole and pyrantel anthelmintics, and more than 95% for macrocyclic lactones, suggest the nematode population is susceptible to the drug tested. Resistance is suspected where reductions of less than 90% for macrocyclic lactones and less than 80% for benzimidazoles and pyrantel are demonstrated. Intermediate values are classified as borderline and tests should be repeated (Vidyashankar et al, 2007).
Both the AAEP and World Association for the Advancement of Veterinary Parasitology recommend herd level testing due to high variability in reduction percentages between individuals (Vidyashankar et al, 2012; AAEP, 2013).
It is also worth noting several studies have demonstrated reduction in apparent efficacy through reduction percentages in youngstock compared to adults on the same farm; therefore, where stock numbers allow, it is most accurate to perform age cohort analyses separately (Relf et al, 2014; Hallowell-Evans et al, 2016).
Where mixed age cohorts are tested, interpretation of results demonstrating overt resistance should be undertaken with caution and repeated testing in all but those cases demonstrating extremely low or negative reductions (increase in FEC over time).
Egg reappearance periods (ERP) are defined as the duration between administration of an effective anthelmintic treatment and resumption of significant strongyle egg shedding (AAEP, 2013). This test is thought to provide an early indicator of the shift in anthelmintic susceptibility of a nematode population towards resistant status as reappearance times shorten post-treatment (Sangster, 2001).
The primary issue with using this technique is the lack of standardisation or wide-scale consensus on definition of “resumption of significant strongyle egg shedding”. Two main definitions are found and used: “the first week post-treatment where a positive egg count (more than 1EPG) is recorded” (Dudeney et al, 2008; Lyons et al, 2008; Molento et al, 2008), and “the time after treatment at which the group arithmetic mean FECs more than 10% of pre-treatment group arithmetic mean FEC” (Borgsteede et al, 1993; Jacobs et al, 1995; Boersema et al, 1996; Mercier et al, 2001; Tarigo-Martinie et al, 2001; von Samson-Himmelstjerna et al, 2007; Larson et al, 2011). The latter definition is most commonly used due to its conservative estimate and relation to the magnitude and spread of FEC data prior to, and two weeks following, treatment; thus providing a more reliable measure of anthelmintic sensitivity at a population level (Matthews, 2014).
This test again relies on faecal flotation diagnostic techniques, so carries the same limitations as sole, herd and FECR tests. The initial step of ERP testing is the FECRT – inclusion criteria for this follow-on diagnostic specifies the initial treatment must have been effective, such as more than 95% reduction following a macrocyclic lactone administration. After confirmation, egg counts continue to be performed for the same individual horses included in the FECRT on a weekly basis until the threshold is exceeded.
This article offers a few key messages. The first is the importance of a representative faecal sample being obtained – this is key for FEC, FECRT and ERP. FEC should be performed on all, or a large proportion of, the herd and interpretation made for the herd. FECs often play a limited role in the diagnosis of clinical parasitic disease as they are often caused by larval stages.