19 Feb 2018
Jacqueline Matthews discusses identification, monitoring and treatment of significant gastrointestinal parasitic helminth burdens.

Image © Alexas_Fotos / Pixabay
Most grazing equids are infected with gastrointestinal parasitic helminths. If animals graze on contaminated pasture and do not receive effective anthelmintic treatment, many worms can accumulate.
The commonest infections affecting equids are members of a group of nematodes known as cyathostomins (small strongyles and small redworms). These parasites have been reported at prevalence rates of more than 90% in many regions, regardless of climatic or management differences.
Anthelmintic resistance is a common problem. Most healthy adult horses carry low burdens of cyathostomins and show no outward signs of disease; however, in some animals – especially young equids – these worms can build up in high numbers (to the order of several million) and cause symptoms ranging from mild weight loss to severe colitis, which can be fatal.
Younger animals are thought to be more likely to develop substantial burdens due to a lack of effective immune responses.
More than 50 cyathostomin species have been described and identified by morphological examination of adult worms – or, in some cases, by using species-specific DNA probes1. High similarity exists in the predominant species catalogued across diverse geographic locations, with Cyathostomum catinatum, Cylicocyclus nassatus and Cylicostephanus longibursatus often the most abundant species.
Although data is limited, studies performed have indicated no association of particular species with pathogenicity or ability to develop anthelmintic resistance. For these reasons – from clinical and control perspectives – the cyathostomin group is treated as a single entity.
Adult cyathostomins are small (less than 2.5cm long), thread-like worms that live in the lumen of the caecum and large colon. High burdens of adult worms can contribute to disease – especially weight loss; however, it is cyathostomin larvae that are associated with severe clinical signs. These are found in the gut mucosa and submucosa, and exist as several “encysted” stages – early third stage (EL3), late third stage (LL3) and developing fourth stage (DL4).
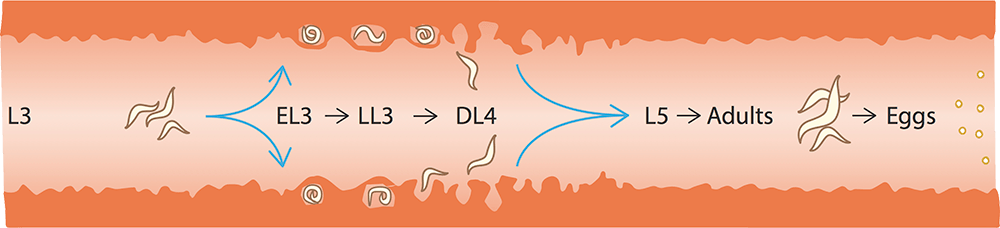
Experimental studies in ponies indicate development time from infection to egg-producing adult cyathostomins varies from 48 days – when a single infection is administered – to 77 days – after repeated multiple infections2. Further studies demonstrate this period can be considerably longer – four months to two years3. This delayed time has been associated with the presence of “inhibited” EL3.
The triggers for larval inhibition are unknown, but the phenomenon has been linked to immunity and environmental factors – such as chilling – to which larvae are exposed prior to infection.
In the UK and other northern temperate regions, encysted larvae are most often found in high numbers in autumn and winter, when they can comprise up to 90% of the total burden4. It is when encysted larvae emerge from the intestinal wall in large numbers that larval cyathostominosis develops.
In the UK, larval cyathostominosis is most often encountered from late autumn to spring. Horses of all ages and breeds can develop the disease, but many cases are younger than five years old or geriatric5. The condition is associated with large intestinal inflammation and impaired gut motility in response to large numbers of larvae emerging from the gut wall.
Clinical signs vary from a vague malaise to sudden onset profuse diarrhoea, severe weight loss and pyrexia. Often, signs are nebulous and include some or all of the following:
Faecal egg counts (FECs) are not a good indicator of larval cyathostominosis, as the syndrome is primarily associated with the presence of larvae that do not release eggs. Indeed, FECs do not correlate strongly with adult burdens. Blood parameters (hypoproteinaemia, hypoalbuminaemia, anaemia, neutrophilia and hyperglobulinaemia) provide non-specific signals.
Close examination of faeces may reveal small, thread-like larvae. As a crude test, faeces can be examined by diluting in water (1:10 to 1:50, depending on consistency) and scrutinising at ×10 magnification.
Often, larval shedding is intermittent or the horse has received an anthelmintic effective at killing luminal stages (adults, emerged larvae) and no larvae are detected. Therefore, reaching a definitive diagnosis can be difficult in practice and vets often depend on “response to treatment” to do so.
No test informs on the level of cyathostomin burden within individuals. FEC tests can be used to detect levels of worm egg shedding into the environment, but FECs do not correlate with the number of worms within horses.
Because no tests enable detection of cyathostomin-encysted larvae, UK recommendations are to treat all horses in autumn/early winter with moxidectin to target these worm stages. In some cases – particularly younger horses – a second moxidectin treatment may be warranted in late winter. Such all-group treatments will have serious implications for selection of anthelmintic resistance.
A cyathostomin-specific diagnostic test is under development at Moredun Research Institute, supported by funding from the Horserace Betting Levy Board, The Thoroughbred Breeders Association and The Horse Trust. Proteins have been identified in cyathostomin larvae that are targets of serum antibody responses in infected horses6,7.
Genes encoding these proteins were cloned from 14 species and the proteins expressed in recombinant form.
A serum ELISA, based on these proteins, identified infected horses within four to six weeks of infection.
A project funded by The Horse Trust is developing a test based on these proteins from the common species C longibursatus, C catinatum and C nassatus. This test will be commercialised in partnership with Austin Davis Biologics. The long-term aspiration is to translate the serum-based test to a saliva format, to enhance uptake in practice.
Encysted cyathostomin larvae – particularly EL3 – are not killed by several broad-spectrum anthelmintics; only fenbendazole (when administered as five daily doses) and moxidectin are licensed against these stages. Fenbendazole resistance in cyathostomins is such that, in many regions, use for this purpose is not recommended8,9.
As aforementioned, moxidectin should be used for its cyathostomin larvicidal properties in autumn/winter. Treatment with this anthelmintic will have the added effect of killing other clinically important worms, such as Strongylus vulgaris larvae.
Treatment protocols are determined by the severity of the signs10. Anthelmintics should be applied in all cases; repeated treatments may be required until signs start to resolve.
Moxidectin is recommended, but care must be taken because of potential toxicity of this lipophilic anthelmintic in thin horses. Symptomatic treatment is important and the protocol applied depends on the individual’s cardiovascular and systemic status.
IV fluid therapy may be warranted – for example, crystalloids (lactated Ringer’s solution and Hartmann’s solution) or colloids (plasma). Close monitoring is essential; electrolyte and acid-base balance should be assessed regularly and, when necessary, adjusted as tissues can flood quickly in severely hypoalbuminaemic individuals. To reduce intestinal wall inflammation, severe cases should be administered with corticosteroid; for example, dexamethasone 0.04mg/kg IV every 24 hours for three to four days.
The potential side effects of corticosteroid treatment must be communicated to the horse’s owners. Some vets advocate application of flunixin meglumine for its anti-endotoxic properties10; however, a low dose must be administered to avoid worsening any worm-associated colitis.
Good nursing is vital – in particular, maintaining appetite and forage intake. The aforementioned protocols are expensive and, sadly, larval cyathostominosis cases often come from resource-poor backgrounds.
In these cases, treatment options are limited and euthanasia should be considered as the prognosis is poor; published studies report mortality rates of 40% to 70% – even with aggressive treatment2.
Prevention is better than cure. Managing pasture larval challenge is key to avoiding cyathostominosis. Effective control needs to take into consideration management, local climatic conditions and the worm life cycle.
It is impossible to eradicate all parasites from all horses; attempting to do so will only select for anthelmintic resistance. Therefore, protocols must seek to minimise infection in the environment using good management, combined with anthelmintic targeting of worm species (large strongyles, small strongyles and tapeworms) and stages (larvae) to prevent disease.
Most adult horses have low worm burdens and egg shedding. This host distribution facilitates protocols that reduce anthelmintic usage across the grazing season by using FEC analysis to inform on treatment requirements of individuals to reduce egg shedding spring to autumn.
Targeted treatment protocols must incorporate good management; dung should be lifted from pasture at intervals that prevent strongyle larvae moving on to grass. In the UK, twice-weekly is recommended in summer.
Basic principles apply – pastures should not be overgrazed and, if “rested”, should be left for a sufficient period to allow substantial death of contaminating larvae. Strongyle third-stage larvae (L3) can survive for six to nine months in cool weather, so local conditions must be considered when calculating how long pastures should be rested before assumed safe.
Alternate/mixed grazing with ruminants can be practised, as most nematode species do not cross-infect; however, liver fluke can develop in ruminants and equids, so care must be exercised if pastures have habitats where snail intermediate hosts of Fasciola hepatica can survive. Harrowing is not recommended, unless pastures are rested for a sufficient time to allow significant L3 mortality.
As aforementioned, encysted cyathostomin larvae do not produce eggs, so are not detectable by FEC tests. Moxidectin should be administered to kill these stages in late autumn/winter. In all cases, when giving advice, a risk assessment of likely helminth transmission should be performed, taking into account the resident population, grazing management, previous anthelmintic use, clinical history, and the results of diagnostic tests.
Every premise presents a unique situation, meaning control protocols should be tailored to each yard.