24 Jul 2017
This article reviews the basics of the physiology and pathophysiology of pain, before focusing on the tools to recognise it in our equine patients.
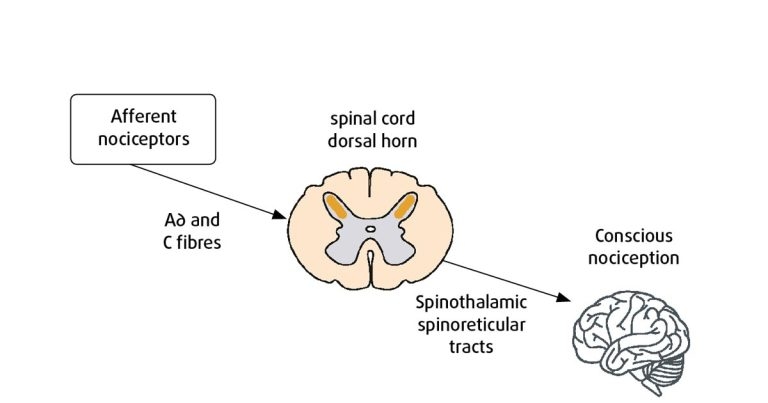
Figure 1. Schematic simplification of ascending nociceptive pathways.
As vets, we see horses in pain on a daily basis, and a large part of our job is to recognise and alleviate that pain.
Pain in animals has been described as “an aversive sensory and emotional experience representing an awareness by the animal of damage or threat to the integrity of its tissues; it changes the animal’s physiology and behaviour to reduce or avoid damage, reduce the likelihood of recurrence and to promote recovery” (Molony and Kent, 1997).
This is the adaptive or physiological response of pain, a protective mechanism to help prevent further injury. On the other hand, “unnecessary pain occurs when the intensity or duration of the experience is inappropriate for the damage sustained, or when the physiologic and behavioural responses to it are unsuccessful at alleviating it” (Molony and Kent, 1997).
This is known as the maladaptive or pathological response. In this case, the response becomes “dissociated” from the original noxious stimuli and requires treatment to minimise the detrimental effects on the horse. Pain is a multidimensional sensory process involving “wide dynamic range” neurones responsive to low and high threshold stimuli (Muir, 2010). The neural pathways are often complex, involving multiple sensory organs, fibre types and areas of the CNS (Muir, 2010).
Figure 1 summarises nociceptive pathways. Peripheral nociceptors respond specifically to noxious or harmful stimuli.
The two main types of nociceptors are high-threshold mechanoreceptors, which respond to mechanical deformation, and polymodal nociceptors, which respond to cell signalling molecules, including pro-inflammatory mediators (prostaglandins; Steeds, 2009).
Two primary afferent/sensory fibre types whose free endings terminate in nociceptors are A∂; small myelinated fibres and C; unmyelinated fibres (Driessen, 2007), which transmit nociceptive information to the spinal cord dorsal horn.
From here, the information is transmitted to supra-spinal locations via ascending pathways, including spinothalamic and spinoreticular tracts. The final destination is the brain; the thalamus processes much of this somatosensory information, from where it is projected to primary and secondary somatosensory cortices and the pre-frontal cortex, where conscious perception of pain occurs (Steeds, 2009).
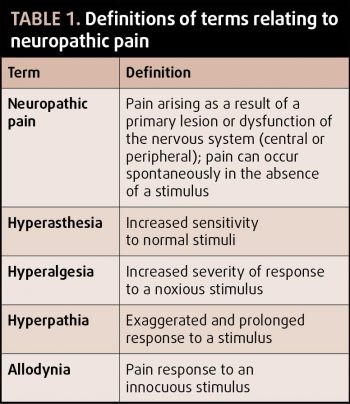
If uncontrolled, nociceptive signalling can lead to persistence and exacerbation of the pain response. The end result is a disproportionate response to the original stimulus, or one that persists beyond cessation of stimuli (see Table 1 for terms and definitions).
The nervous system is adaptable and its response to stimuli can change over time – a concept known as plasticity, which occurs through the process of sensitisation.
Peripheral sensitisation (primary hyperalgesia) occurs following injury or insult, with changes in the local chemical environment in surrounding nociceptors. This results in activation and sensitisation of peripheral nociceptors to noxious stimuli (hyperalgesia) and potentially non-noxious stimuli (allodynia).
Central sensitisation (secondary hyperalgesia) results from continuous nociceptive input to the spinal cord, resulting in up-regulation of release of excitatory neurotransmitters within the dorsal horn. Although the mechanisms are not well defined, the N-methyl D-aspartate (NMDA) receptor is thought to play a key role via increased glutamate sensitivity (Rison and Stanton, 1996). The end result is the phenomenon of “wind-up”, in which the neurones within the dorsal horn become increasingly responsive to noxious stimuli. The neuronal network subsequently undergoes morphological changes as a response to persistent, high-level afferent input.
Both peripheral and central sensitisation have been demonstrated in the horse (Spadavecchia et al, 2004), with death often the outcome, even when the inciting cause can be successfully treated. This highlights the importance of pain management from a pathological perspective, in addition, of course, to preservation of animal welfare.
Pain has been divided into three categories based on the physiological or pathophysiological processes underlying.
The results of the physiological process of nociceptive signalling, usually sharp, localised and affecting a well-defined area. This is evoked by high intensity stimuli and is adaptive and brief. Examples include needle pricks and responses to hoof testers.
This is evoked by high and low-intensity stimuli secondary to tissue injury. Sensitisation often occurs. This pain is adaptive and reversible and continues until inflammation decreases. Examples include lacerations, synovitis, surgery, tendonitis and desmitis.

Maladaptive and persistent pain, evoked by high and low-intensity stimuli. Central sensitisation is sustained and pain can occur in the absence of continued noxious stimui. Examples include nerve trauma and
neuroma formation.
Identifying pain in our equine patients can be difficult. Without verbal communication, we are relying on the accurate identification of obvious behavioural or locomotor abnormalities and interpretation of physiological indices. Clearly, when a horse is demonstrating non-weight bearing lameness or violent signs of abdominal discomfort, whether the horse is in pain is not in dispute. But what about the more subtle signs?
As horses are a prey species, they may not overtly demonstrate pain in a situation they perceive to be dangerous. Also, breed and individual variations in expression of pain can vary greatly. This can make interpretation of pain somewhat difficult.
Nevertheless, behavioural and physiological assessments are the mainstay, and some research in the past few years has started to help us refine our assessment of equine pain.
More often than not, musculoskeletal pain manifests as lameness or impaired locomotion. We assume the degree of lameness exhibited by the horse correlates with the degree of pain (Schatzmann and Spadavecchia, 2004).
Many ways to subjectively (American Association of Equine Practitioners lameness scale, composite orthopaedic pain scale) and objectively (Lameness Locator, force plate analysis) assess lameness are available. Generally, musculoskeletal pain, if producing locomotor abnormalities, is relatively easy to identify and assess – albeit some lameness is very subtle.
Pressure algometry (PA), a mechanical form of pain assessment, has started to be used in horses, and shows some promise in the objective determination of musculoskeletal pain (back pain) in horses (Haussler and Erb, 2003). This study used a handheld instrument to quantify mechanical nociceptive thresholds (MNTs), thus its clinical applications are to provide objective assessment of nociception and to aid in localising pain to affected structures.
MNTs have been described in normal horses (Haussler and Erb, 2003), but little work has been conducted in naturally occurring disease. One study used PA to compare MNTs before and after experimentally induced pain, concluding nociceptive thresholds were considerably lower after induction of pain – indicating hyperalgesia at the sites of pain (Haussler and Erb, 2006).
An increasing number of studies are evaluating equine pain, with certain behavioural changes being consistently observed in horses experiencing certain types of pain (acute versus chronic, musculoskeletal versus visceral). Although this information is vital in terms of identifying pain, we must not forget we have much still to learn about equine behaviour, and also many factors will influence it, such as environment, temperature and hunger. Individual variations can be dramatic, so information from the owners and trainers regarding an individual’s “normal” behaviour is invaluable (Zarucco, 2007).
Guedes (2017) suggested: “In general, horses in pain will express new and unusual behaviours and stop expressing normal behaviours, spontaneously or evoked. These behaviours may or may not be accompanied by changes in physiologic parameters, such as heart rate, respiratory rate, blood pressure and gut motility. These parameters should be normalised by adequate analgesic strategies.”
Various scoring systems have been suggested in an attempt to improve our pain detection capabilities through repeated or continuous visual behavioural assessment. One such example comes from Lindegaard et al (2010a; Table 2). This scale was designed to evaluate the use of intra-articular morphine following experimentally induced synovial sepsis, but has been successfully modified and used to evaluate other types of pain.
Another group has suggested humans have very well-developed skills for identifying emotion or pain through facial expressions. Moreover, evidence suggests facial expressions are a reliable indicator of pain in a variety of animals, including mice (Langford et al, 2010), rats (Sotocinal et al, 2011) and rabbits (Keating et al, 2012).

In horses, kinematic analysis has been used to demonstrate facial expressions change in response to injection (Love et al, 2011), and further work has supported this finding, with the development of a Horse Grimace Scale in horses undergoing castration (Dalla Costa et al, 2014). Figure 2 demonstrates changes in ear positioning, tension around the orbital region, prominent masticatory muscles, chin position and nostril flattening to be associated with increased composite pain scores.
Similarly, this change in facial expressions has been induced experimentally through application of noxious stimuli and observations of responses, yielding the development of the term “an equine pain face” consisting of low and/or symmetrical ears, an angled appearance of the eyes, a withdrawn and/or tense stare, mediolaterally dilated nostrils and tension of the lips and facial muscles (Gleerup et al, 2015; Figure 3; Table 3).
Although many of these parameters are subjective, they are seemingly repeatable among studies and, although much work is still to be done, these features hopefully assist in the detection of more subtle signs of pain that might otherwise go unnoticed in our patients.
The premise of all analgesic plans should be based on providing pre-emptive and multimodal analgesia.
Clearly, pre-emptive analgesia – that is, any given before the noxious stimulus – is not always applicable, as we often see our patients because they are perceived to already be in pain. However, pre-emptive analgesia is very important with regards to elective surgical procedures.
A variety of drugs and drug combinations may be chosen for this purpose, the important point being the medication is provided before the noxious stimulus, with enough time so the medication can be administered and have taken effect.
Multimodal (balanced) analgesia refers to the concept of using two or more analgesic mediations with different mechanisms of action, targeting different parts of the afferent nociceptive pathways, with the aim of producing additive or synergistic effects and reducing the side effects of any one substance.
Therefore, the six main aims in a balanced analgesia protocol (Driessen, 2007) are:
The concept of multimodal analgesia also encompasses the use of complementary modalities, including acupuncture and chiropractic therapy.

These include procaine, lidocaine, mepivacaine and bupivacaine. Their mechanism of action is via sodium ion channel blockade, therefore prevention of action potential propagation in A∂ and C fibres, thus resulting in local desensitisation.
Lidocaine (2%) can be used as an IV infusion at an initial bolus of 1.3mg/kg over 10 to 15 minutes, followed by a continuous rate infusion at 0.05mg/kg/min in standing or anaesthetised horses. Although not licensed for this use, lidocaine in this capacity has demonstrated analgesic, anti-inflammatory and indirect prokinetic properties, although the exact mechanisms remain unknown.
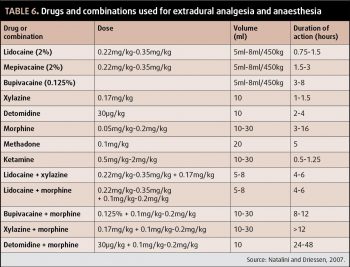
Local anaesthetics can also be used locally, either in wound catheters or via distal limb catheters, for administration of continuous perineural analgesia – a technique described by Zarucco (2007). Intra-articular and extradural routes are also useful (Table 6).
Opioids produce their analgesic effects through interaction with the μ and kappa opioid receptors.
The μ-opioid receptor is thought to be the most significant in terms of analgesia, hence full μ-opioid agonists are likely the most potent analgesics (morphine, methadone, fentanyl and pethidine).
Morphine is the gold standard analgesic others are compared to. Although not licensed, it is commonly used under the cascade for analgesia and pre-anaesthetic medication. As with most opioids, the main routes of systemic administration are IV and IM. Oral bioavailability is low, so this route of administration is not useful.
Full μ-opioid agonists are reported to exhibit a dose-dependent increase in muscle tone and locomotor activity due to dopaminergic effects (Combie et al, 1981), and higher doses may cause euphoria/dysphoria or hyperexcitability.
This effect is likely less pronounced with kappa-opioid agonists. Combination of opioids with an alpha-2 agonist helps prevent the excitatory effects and potentates the sedative action.
Butorphanol is a μ-opiod antagonist and a kappa-opioid receptor agonist. Although theoretically a potent analgesic, pharmacological studies have demonstrated a ceiling effect – that is, increasing the dose doesn’t increase analgesic effects.
Newly licensed buprenorphine is a partial μ-opioid agonist, demonstrating excellent receptor affinity, but only partial agonist activity, once bound. It has shown some promise as a twice-daily analgesic in horses, with some evidence to demonstrate superior analgesic effects to that of butorphanol (Love et al, 2011). Pharmacokinetic studies have demonstrated analgesic concentrations present in plasma 30 minutes after IV administration of 0.005mg/kg, although higher doses may be required for IM use (Davis et al, 2007).
CNS excitement and decreased gastrointestinal motility has been noted, particularly with IV use. The excitement, as mentioned before, can be avoided with concurrent alpha-2 agonist administration.
Sublingual bioavailability has also been shown to be good, with similar plasma concentrations to IV use in one study (Messenger et al, 2011).
Pethidine is licensed in horses and must not be administered IV because of the potential for histamine release, resulting in severe hypotension, excitement and potentially collapse.
Concerns regarding decreased intestinal motility with opioid use are controversial. It is generally accepted single perioperative doses are unlikely to cause a problem, while high doses or repeated or prolonged use may be associated with decreased motility and the development of gastrointestinal impactions. Evidence is, however, limited and should be investigated further.
Opioids may also be administered locally. Intra-articular morphine has been shown to have analgesic (Lindegaard et al, 2010a) and anti-inflammatory (Lindegaard et al, 2010b) effects, with treated joints having lower total protein and serum amyloid A concentrations in experimentally induced synovitis.
Opioids are commonly administered via the extradural route. “Caudal epidural anaesthesia” is useful for analgesia and desensitisation of the rectum, vulva, perineum, urethra and bladder, with the aim of desensitising these structures without loss of motor function to the hindlimbs (Natalini and Driessen, 2007).
Preservative-free formulations should be used for administration into the extradural space.
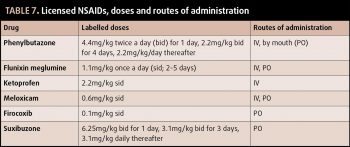
NSAIDs are perhaps the systemically administered analgesics we would all reach for in the first instance. Licensed NSAIDs in horses include phenylbutazone, flunixin meglumine, ketaprofen, meloxicam, firocoxib and uxibuzone (Table 7). Labelled doses are often for once-daily administration, but are commonly given twice daily to provide adequate analgesia.
Concerns regarding side effects (gastrointestinal and renal) mean these drugs are perhaps best used conservatively in at-risk patients. The main potential adverse effects are gastrointestinal (gastrointestinal ulceration and right dorsal colitis) and renal pathology. High doses for prolonged periods and combinations of NSAIDs administered concurrently should be avoided for this reason.
One study demonstrated severe adverse effects in all horses administered a combination of phenylbutazone (2.2mg/kg by mouth every 12 hours) and flunixin meglumine (1.1mg/kg IV every 12 hours) for five days, including severe hypoproteinaemia, hypoalbuminaemia and colitis, necessitating euthanasia in one horse.
Horses treated with phenylbutazone alone did not have significantly different protein or albumin concentrations compared with controls (Reed et al, 2006). Many horses on the combination treatment also developed significant gastric ulceration.
Much effort has gone into investigation of the use of cyclooxygenase (COX)-2 selective NSAIDs in horses, such as firocoxib, in an effort to reduce adverse effects associated with decreased prostaglandin production on renal and gastrointestinal blood flow. A clear benefit to the use of COX-2 selective drugs has not been demonstrated in horses. Hence choice of drug often depends on availability and clinician preference.
Caution should be exercised in using these drugs due to the risk of adverse effects and lower doses should be used for the least time necessary. Treatment should be discontinued if any gastrointestinal side effects are noted, and serum albumin concentrations should be examined if there is cause for concern.
A dissociative anaesthetic, ketamine is widely used as an anaesthetic induction agent in horses. Its NMDA-receptor antagonism makes it a potent analgesic, as well as an inhibitor of central sensitisation for the same reason.
A variety of routes of administration have been used. Systemically, ketamine can be administered as a CRI at dose rates of 0.4mg/kg/hr to 1.5mg/kg/hr. No severe adverse effects have been noted, but care should be taken to avoid accidental overdose resulting in hyperexcitability and anaesthesia. Therefore, infusion pumps must be used to ensure more accurate dose delivery.
Ketamine has also been used locally, including in extradural preparations (Table 6), as well as perineural and intra-articular administration. Perineurally, ketamine
results in analgesia of short duration (around 15 minutes; López-Sanromán et al, 2003), hence its usefulness clinically as a single dose is questionable.
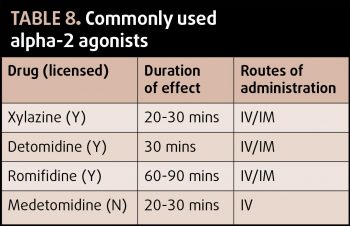
Commonly used for standing restraint, the alpha-2 agonists also provide potent analgesia. Three licensed products are available, with xylazine being the shortest acting and romifidine being the longest. IV administration may consist of bolus injections of CRI, which may be useful in both anaesthetised and conscious horses. Adverse effects include sweating, increased urine production (due to osmotic diuresis and anti-diuretic hormone antagonism), bradycardia and atrioventricular block, upper airway obstruction in horses with upper airway disease and ileus, so patient selection is important.
Gabapentin is a human drug used in the management of neuropathic pain and as an anti-seizure medication. It has been used in horses for management of neuropathic pain (for example, trigeminal neuritis and femoral neuropathy) with some success (Davies et al, 2007).
It can be administered IV or by mouth, although oral bioavailability is low (Terry et al, 2010), which may limit its usefulness away from a hospital setting. Oral doses include 2.5mg/kg at intervals of 8, 12 or 24 hours (Davis et al, 2007) and 2mg/kg to 3.3mg/kg at intervals of 8 or 12 hours (Dutton et al, 2009).
Efficacy is largely judged by improvement of clinical signs (for example, improvement in head-shaking frequency or severity, improved pain scores). Behavioural effects include increased sedation, as well as increased drinking frequency, and are more commonly associated with IV use.
Limited literature is available for the duration of treatment and this will depend on the disease process. In the case report by Davies et al (2007), postanaesthetic femoral neuropathy was treated for six days with gabapentin; however, more protracted courses may be required for head shaking.
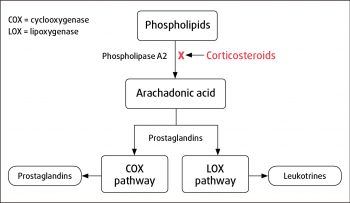
Potent anti-inflammatory medications, corticosteroids provide effective relief from inflammatory pain. Figure 4 demonstrates the overall mechanism of action, resulting in decreased arachadonic acid release from membrane phospholipids.
A common analgesic application is the use of intra-articular cortiocosteroids for the treatment of arthritis and synovitis. Many beneficial effects of intra-articular corticosteroids have been demonstrated, including decreased inflammatory cytokine and matrix metaloproteinase concentrations.
However, some chondrodestructive effects have been observed, more commonly with methylprednisolone acetate, including chondrocyte necrosis and reduced cartilage matrix synthesis (Goodrich and Nixon, 2006). These effects are less apparent with triamcinolone, where extracellular matrix production is not negatively affected (Richardson and Dodge, 2003).