26 Feb 2018
Andy Durham takes a look at this equine disease, and management and preventive strategies.

Laminitis remains a common and debilitating equine disease affecting about 1 in 15 horses and ponies annually, according to the National Equine Health Survey (Slater, 2017).
Despite advances in understanding the causes of laminitis increasing our ability to prevent it, much resistance exists to implementing the required preventive strategies.
Indeed, a resigned acceptance often appears among owners that laminitis is an inevitability in certain breeds and types, with throwaway comments such as “he is always a bit footy in the spring”. The same owners may not be so dismissive of an unfit, overweight human complaining of occasional chest pain.
Recognition of laminitis, especially by owners, may not always be easy.
Advanced clinical cases may be relatively obvious, demonstrating fairly specific signs – such as reluctance to move or have a limb lifted, walking with preferential landing and loading of the heel area, and additional pain evident while turning or transitioning from soft to hard surfaces.
Hoof temperature may be variable and subjective, although a more easily palpated digital artery pulsation is a fairly sensitive finding, especially after walking a few steps. However, laminitis is frequently subclinical.
Many research studies have discovered, unexpectedly, quite marked radiographic changes in horses’ feet while screening for laminitis, despite an absence of a clinical history.
Farriers are often the first to note hoof changes – such as expansion or haemorrhage in the white line, or abnormal hoof rings – and this should always be taken very seriously, even when no evidence of hoof pain exists.
In the author’s opinion, the biggest general advancement in veterinary medicine in the past few decades has been the ability to detect and intervene with many diseases at an earlier clinical, or subclinical, stage. This offers obvious advantages to the affected individual and we should never ignore signs of disease, however early or mild they may be. Preventive and early therapeutic interventions will, inevitably, be more successful than trying to fix more severely damaged hoof and foot structures.
Research has greatly increased our understanding of the causes of laminitis and consequent preventive approaches. We have a clearer understanding laminitis may be caused by three distinct types of inciting factors (Panel 1).
Toxaemic laminitis may result from septic conditions, such as colitis, pneumonia or metritis, but is relatively uncommon and may be effectively countered in the early stages by cryotherapy, as pioneered by Andrew van Eps (van Eps, 2010; van Eps and Orsini, 2016; Figure 1).
Load-bearing laminitis is also quite rare, but may be seen in hospitalised horses with severe lameness in the contralateral limb (for example, following fracture fixation). This form of laminitis is especially concerning and creates severe challenges in prevention and treatment. However, published studies and clinical experiences indicate, by far, the commonest form of laminitis occurs in association with endocrinopathies – comprising equine metabolic syndrome (EMS), pituitary pars intermedia dysfunction (PPID; equine Cushing’s disease) and, occasionally, exogenous administration of glucocorticoids (Karikoski et al, 2011; Donaldson et al, 2004).

Animals susceptible to endocrinopathic laminitis will frequently respond differently to normal animals when ingesting feedstuffs high in non-structural carbohydrates (NSC), such as pasture or cereal. Given the high frequency of endocrinopathic forms of laminitis, strategies to foresee and prevent such episodes should be emphasised in discussion with owners of susceptible animals.
The following summarises the approach to diagnosing endocrinopathic laminitis in the horse, although more information is freely accessible as downloadable information sheets and abstracts from specialist meetings or as podcasts (see further reading).
Increased risk of laminitis can be identified by looking for known risk factors of the condition. These may comprise a combination of historical and clinical features, as well as laboratory test results.
EMS describes the collective presence of risk factors for laminitis, including insulin dysregulation. Certain breeds/types may be more likely to be affected by EMS than others, including native ponies, cobs and Warmbloods, although any horse may develop EMS if not managed correctly.
Generalised or regional obesity and predisposition to weight gain is common, but not always present. Additional features may include cardiovascular changes, including increased blood pressure and adipose dysregulation manifesting as abnormal adipokine concentrations, especially low high molecular weight (HMW) adiponectin.
Insulin dysregulation is a central feature of EMS and must be identified to satisfy the diagnostic criteria for EMS.
Three general test types are used to indicate insulin dysregulation (Panel 2). In practice, tests aimed at identifying insulin resistance are not popular as they inevitably involve sequential blood testing after exogenous insulin injection (for example, insulin response test or combined glucose insulin test).
Measurement of basal insulin concentrations (allowing hay access, but avoiding any hard feed for several hours beforehand) may be useful, as when hyperinsulinaemia (typically more than 20mU/L) is found then this is indicative of insulin dysregulation. However, most cases of EMS will have normal basal insulin concentrations, rendering negative results uninformative.
Although measuring insulin following a period of fasting has been advocated in the past, expert consensus no longer supports this as a diagnostic test due to lack of sensitivity and specificity. In practice, the most accurate and practical means of establishing insulin dysregulation is to measure serum insulin concentration between 60 and 90 minutes following oral dosing with 45mL corn syrup per 100kg bodyweight. This test does not require a period of fasting beforehand.
An excessive insulin response to corn syrup not only identifies insulin dysregulation and increased risk of laminitis, but also directly implies the need for a diet low in non-structural carbohydrates (sugars, starches and fructans) so dangerously high postprandial insulin spikes are reduced.
It is very important to remember insulin values can be markedly different on different analysers, and it is the responsibility of each individual laboratory to establish their own reference intervals by a valid means.
Adipokines are a group of peptides secreted by fat cells that have generated considerable interest in human and equine metabolic disorders. It is evident in EMS not all fat horses get laminitis and not all laminitis cases are fat. Therefore, the metabolic activity of the fat appears to be more important than the absolute amount of fat present.
Although several equine research studies have examined serum leptin concentrations, the general impression is this adipokine simply reflects fat mass in the horse, rather than the metabolic status of the individual. In contrast, adiponectin appears to better reflect metabolic status and laminitis risk in horses and ponies (Bamford et al, 2016; Menzies-Gow et al, 2017). Adiponectin is secreted by fat cells as a 30kDa monomer, which then forms complexes of various sizes.
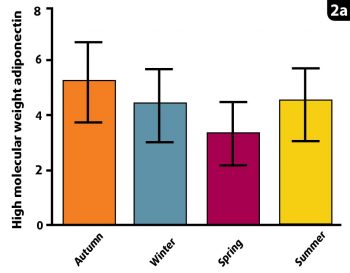
In all species studied, it is evident the HMW multimers are the metabolically active fraction that correlates best with metabolic status. Indeed, impaired multimerisation – affecting HMW, but not total adiponectin – is a key feature of metabolic diseases (Wooldridge et al, 2012). Although adiponectin is secreted by fat, it is actually a beneficial product, which improves insulin regulation. However, when obesity develops, a decrease in serum HMW adiponectin fractions (although not necessarily total adiponectin) exists, which may contribute to obesity-related insulin dysregulation. Both equine HMW adiponectin and total adiponectin assays were first validated and investigated at the Liphook Equine Hospital, although HMW adiponectin is preferred, as it has the strongest scientific support.
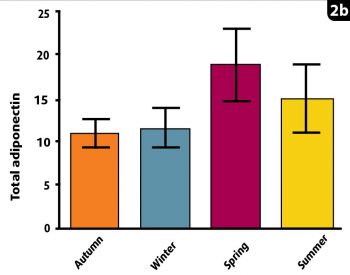
A study by Liphook Equine Hospital and the RVC compared seasonal changes in total and HMW adiponectin. This revealed the lowest HMW adiponectin concentrations were in the spring, consistent with the highest laminitis risk at that time. In contrast, total adiponectin concentrations were actually at their highest in spring, incorrectly suggesting the lowest laminitis risk (Figure 2). Therefore, HMW and total adiponectin are not always well correlated with one another in horses, as in other species.
PPID may occur independently or coexist with EMS. PPID indicates abnormal secretion of multiple peptides from a hyperplastic, hypertrophic or adenomatous pars intermedia. A variety of clinical signs may ensue, including laminitis.
Although PPID is more common in older horses (20% of those more than 15 years old; McGowan et al, 2013), more rarely, horses as young as seven may be affected (Orth et al, 1982).
Of additional importance is the observation many confirmed cases of PPID present only with signs of laminitis and may not show other signs of the condition, such as abnormal hair and coat, and polydipsia.
Therefore, it may be hard or impossible to exclude PPID on clinical grounds when faced with a case of laminitis.
Measuring basal plasma adrenocorticotropic hormone (ACTH) remains the preferred and most convenient means of identifying PPID. When compared with postmortem confirmation of disease, basal ACTH has excellent specificity (98%; that is, normal horses are very unlikely to test positive), although may lack sensitivity (84%; that is, some horses with PPID will have normal ACTH; van der Kolk et al, 1995; Beech et al, 2007; Beech et al, 2011).
Therefore, where clinical suspicions of PPID remain, despite a normal basal ACTH concentration, it is recommended to perform a thyrotropin releasing hormone (TRH) stimulation test.
PPID cases are expected to have ACTH concentrations greater than 200pg/mL at 10 minutes following an IV bolus of 1mg TRH (can be obtained from the Liphook Equine Hospital Laboratory; [email protected]).
Step one is to identify the causes of laminitis as discussed above. Step two is to control or eliminate the risk factors.
Failure to directly tackle the risk factors will inevitably lead to recurrent laminitis. As Henry Ford often said, “if you always do what you’ve always done, you’ll always get what you always got”.
The key elements of EMS control are to improve insulin regulation via nutrition and improved aerobic fitness. The most important dietary approach is limitation of non-structural carbohydrate (NSC) consumption (Bamford et al, 2016) via selection of low NSC feedstuffs (no cereal content and limited to absent grazing) and, possibly, removal of a proportion of water-soluble carboydrates (sugars and fructans) by soaking forage.
If judged to be overweight, calorie restriction is also important by limiting daily food intake to between 1.2% and 1.6% bodyweight as fed. Increased exercise may help burn calories and have a possible direct benefit on insulin regulation.
There is no way to avoid facing the realities of dietary and weight control as outlined, and drugs should never be an alternative to this.
However, at least two drugs have been shown to encourage and support weight loss and improve insulin regulation alongside dietary control (Durham, 2017).
Metformin (15mg/kg to 30mg/kg) has been used effectively to help control insulin dysregulation with specific effects demonstrated by limiting postprandial glucose absorption and hyperinsulinaemia. Additionally, levothyroxine at 0.1mg/kg may be considered to increase metabolic rate and weight loss, alongside improving insulin regulation, but it is very costly in the UK.
When PPID has been diagnosed in a laminitic individual, pergolide therapy is almost always indicated. Starting at a dose of 0.5mg for a pony and 1mg for a horse, this drug is highly effective in improving clinical signs and plasma ACTH in the majority of individuals within a few weeks of starting treatment (Durham et al, 2014).