10 Sept 2018
Nicola Menzies-Gow discusses the two common disorders of this condition. focusing on diagnosis, clinical signs and management options.

Figure 1. A 10-year-old Welsh pony mare with generalised obesity.
Two endocrine disorders commonly affect the horse: equine metabolic syndrome (EMS) and pituitary pars intermedia dysfunction (PPID).
EMS is a collection of risk factors for endocrinopathic laminitis. Insulin dysregulation (ID) is the central feature and additional features include obesity (generalised or regional), cardiovascular changes and adipose dysregulation. Diagnosis of EMS centres on demonstration of ID using basal and/or dynamic tests. Management consists of dietary modification, exercise and use of pharmacologic agents.
Other far less common equine endocrine disorders include diabetes mellitus (DM), diabetes insipidus (DI), disorders of the thyroid gland and adrenocortical dysfunction. Clinical signs associated with DM include weight loss despite polyphagia, polydipsia (PD) and polyuria (PU). A diagnosis of DM is made when hyperglycaemia occurs with concurrent demonstration of ID and pancreatic beta cell dysfunction. DI is a rare disorder of horses characterised by moderate to severe PU/PD and a diagnosis is made based on a water deprivation test. The most common disorder of the equine thyroid gland is neoplasia or hyperplasia.
In most cases thyroid enlargement is the only reported clinical sign; however, occasionally thyroid tumours have been associated with hyperthyroidism. Clinical signs of hyperthyroidism include weight loss, tachycardia, tachypnoea, hyperactive behaviour, ravenous appetite, alopecia and cachexia. The prevalence of primary hypothyroidism in adult horses is very low; clinical signs include lethargy, exercise intolerance and poor coat hair.
Finally, adrenocortical dysfunction can manifest as either abnormal increases or decreases in activity. Primary hyperadrenocorticism and permanent hypoadrenocorticism (Addison’s disease) are rare in horses. However, hyperadrenocorticism may occur secondary to PPID and transient reversible adrenocortical dysfunction, resulting in cortisol insufficiency, can develop during critical illness.
Two endocrine disorders that commonly affect horses are equine metabolic syndrome (EMS) and pituitary pars intermedia dysfunction (PPID). Only the former will be covered in this article.
In addition, several other equine endocrine diseases have been described and occur rarely.
The key central and consistent feature is insulin dysregulation (ID), which may manifest as basal (resting) hyperinsulinaemia, an excessive insulin response to an oral or IV carbohydrate challenge and/or tissue insulin resistance.
Further inconsistent features of EMS comprise obesity, which may be generalised (Figure 1) or regional (Figure 2); cardiovascular changes, including increased blood pressure, heart rate and cardiac dimensions; and adipose dysregulation manifesting as abnormal plasma adipokine concentrations, including hypoadiponectinaemia and hyperleptinaemia.
It is well established that hyperinsulinaemia induces laminitis in horses. Field-based studies have shown an association between hyperinsulinaemia, or other components of ID, and laminitis and experimental studies demonstrate laminitis can be induced by 48 to 72 hours of insulin infusion in ponies and horses. However, exactly how hyperinsulinaemia causes laminitis remains to be determined.
Theories, including glucose deprivation, glucotoxicity, matrix metalloprotease upregulation and increased blood flow-induced hyperthermia, have been disproven. Other theories include endothelial dysfunction, resulting in vasoconstriction, inappropriate signalling via insulin-like growth factor receptors, and microbiota and gut permeability changes, although these have not been proven.

The expression of EMS is the result of a complex interaction between genetics and environment. Although environmental factors, including excessive nutrition, have been linked to EMS, high planes of nutrition and/or changes in the pasture do not result in metabolic derangements and laminitis in all horses, indicating the importance of underlying genetic influences.
Horses with EMS are often considered to be “good doers” or “easy keepers”, and genes contributing to this phenotype are likely to be advantageous for survival in the wild during periods of famine by enhancing feed efficiency. Initial research found the prevalence of laminitis was consistent with the action of a major gene or genes expressed dominantly, but with reduced penetrance attributable to sex-mediated factors, age of onset and further epigenetic factors.
Genome wide association studies using Arabian horses, Welsh ponies and Morgan horses have identified several candidate genes associated with relevant traits, including height and insulin, triglyceride and adiponectin concentrations. Research is ongoing.
Little epidemiological data exists relating to the prevalence of EMS, although the prevalence of some of its components has been evaluated. For example, the reported prevalence of hyperinsulinaemia ranges between 18% and 27%.
Published cases of EMS largely involve British native breed ponies, and cases of primary endocrinopathic laminitis were more likely to occur in British native ponies compared to Nordic ponies, cold-blooded horses, and warm-blooded and hot-blooded horses in one Finnish study1. The prevalence of obesity ranges from 21% to 45% in the UK and 8% to 51% in other countries.
Only one study has examined regional adiposity, with the prevalence of a cresty neck score more than three out of five being 33% in horses in south-west England2. Draught-type, cob-type, British native and Welsh breeds, Shetland ponies, Rocky Mountain horses, Tennessee walking horses, quarter horses, warmbloods and mixed-breed horses were more likely to be obese compared to Thoroughbreds.
Finally, animals described as “good doers”, those used for pleasure riding, non-ridden animals and more dominant ones were more likely to be obese.

By definition, laminitis (Figure 3) is the primary clinical consequence of EMS. However, horses with EMS might also be at risk of further problems, including hyperlipaemia and critical care-associated metabolic derangements, such as hyperglycaemia and hypertriglyceridaemia.
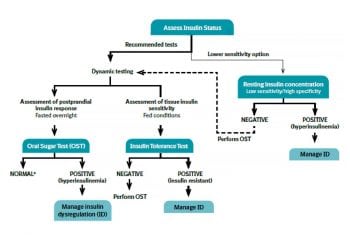
A diagnosis of EMS is based on demonstration of ID (Figure 4).
The differential diagnoses for EMS are:
The management of EMS consists of dietary modification, exercise and the use of pharmacologic agents.
Dietary modification recommendations depend on whether the animal is obese or lean.
In horses with weight-loss resistance, a further forage restriction to 1% BM as DMI or 1.15% BM as fed may be considered if appropriately monitored. Grains or cereal-based feeds should be excluded due to their high content of non-structural carbohydrate (NSC), and high-fat feeds should be avoided due to their high energy content. Additionally, fruit or vegetables, such as carrots, apples or treats with a high glycaemic load, should be avoided.
The nutrient composition of the forage should be determined where possible and hays with low NSC content (fewer than 10%) are recommended to limit postprandial insulin responses. Soaking is advised to reduce the NSC content of the hay if necessary and as forages can be low in protein, and mineral and vitamin leaching occurs after soaking, so these nutrients must be balanced by low calorie supplements to cover requirements.
During the initial 6 to 12 weeks of dietary restriction, pasture access should be prevented as even partial access is very difficult to quantify. However, successful long-term management of EMS cases can still include some grazing provided that ID, especially assessed by the insulin response to oral carbohydrates or grazing, is under control and that grazing is also carefully controlled.
The use of dietary supplements, such as cinnamon, magnesium and chromium, to facilitate weight loss or to improve ID is popular, but their efficacy remains questionable or unproven.
Diabetes mellitus (DM) is defined as a persistent hyperglycaemia from reduced insulin secretion and/or insulin resistance. Type-one DM is the term used to describe DM resulting from pancreatic β-cell destruction, with decreased circulating insulin concentrations.
Primary DM in the horse is rare and appeared to result from chronic pancreatitis, which was attributed to large strongyle larval migration in one case. Type-two DM results from insulin resistance and gradual onset pancreatic β-cell failure. Most cases of equine DM develop as a consequence of PPID, possibly due to antagonism of insulin by cortisol and other pars intermedia-derived hormones, and most likely to represent type-two DM.
Reported clinical signs include weight loss, despite polyphagia, polydipsia (PD) and polyuria (PU).
A diagnosis of DM is made when hyperglycaemia occurs with concurrent demonstration of ID and pancreatic beta cell dysfunction using minimal model analysis of a frequently sampled IV glucose tolerance test. Hypertriglyceridaemia may also occur.
The long-term management of insulin-dependent DM in a horse with auto-immune polyendocrine disease has been described, and included dietary modification and administration of an intermediate-acting insulin once daily.
Treatment of type-two (non-insulin-dependent) DM involves dietary modification to a low glycaemic ration, characterised by high fibre content, low NSC and added calories in the form of fat. Although both metformin and glyburide have been advocated to promote insulin action in the management of DM in horses, the scarcity of the condition has limited the opportunity to assess their clinical usefulness.
Metformin is potentially helpful because it inhibits hepatic glucogenesis and fatty acid oxidation, reduces intestinal absorption of glucose, and enhances tissue insulin sensitivity. However, the oral bioavailability of metformin in horses appears to be low. Glyburide may be helpful because it promotes the release of endogenous insulin from β-cells.
Diabetes insipidus (DI) is a rare disorder of horses characterised by moderate to severe PU/PD. PD in adult horses can be defined as water intake more than 100ml/kg daily; typical water intake for horses is 40ml/kg to 60ml/kg daily. PU is defined as urine production more than 50ml/kg daily; normal urine production is typically between 15ml/kg and 30ml/kg daily. DI can be divided into neurogenic and nephrogenic DI.
Neurogenic DI is characterised by a lack of production of antidiuretic hormone (ADH) by the pituitary gland, secondary to hypothalamic neuronal degeneration. This degeneration may be congenital (such as cerebral malformation and hypoxic-ischaemic encephalopathy) or acquired (secondary to trauma, infection and idiopathic) and a handful of cases of each have been described in the horse.
Nephrogenic DI results from the failure of ADH activity on cortical and medullary collecting ducts. Only a congenital form has been rarely described in the horse.
The water intake and, ideally, urine output should be monitored to demonstrate the presence of PU/PD.
Firstly, it is important to rule out physiologic explanations, such as hot weather, hard work, lactation, excessive dietary protein and/or excessive salt consumption, administration of glucocorticoids or diuretics. Secondly, other more common pathologic causes of PU/PD should be excluded.
If DI is suspected, a water deprivation test – whereby water is withheld for 12 to 24 hours or a modified water-deprivation test whereby water is withheld for 12 to 24 hours after 3 to 5 days of limiting water intake to about 75ml/kg/day, with bodyweight monitoring to prevent losses exceeding 5% – should be undertaken. Failure for the urine specific gravity (SG) to increase more than 1.02 is consistent with a diagnosis of DI.
Further tests to distinguish neurogenic and nephrogenic DI include measurement of serum ADH concentrations at the end of the water deprivation test or the ADH response test (inject 0.05μg/kg desmopressin acetate IV and measure urine SG over 24 hours; SG under 1.02 indicates nephrogenic DI).
The differential diagnoses for PU/PD include renal insufficiency, PPID, psychogenic polydipsia, excessive salt consumption, diabetes mellitus, sepsis and/or endotoxaemia, and iatrogenic causes (sedation with α2-agonists, corticosteroid therapy, or diuretic use).
In other species, neurogenic DI is treated with hormone replacement therapy and nephrogenic DI is treated with dietary sodium restriction and administration of thiazide diuretics. However, long-term therapy of DI has not been described in the horse.
The thyroid gland is a bilobed organ located dorsal to the trachea, just distal to the larynx. This gland is composed of cuboidal to low columnar epithelial cells that produce thyroglobulin. Iodine is bound to the tyrosine residues within thyroglobulin to form thyroxine and triiodothyronine (T3).
Thyroid hormone secretion is stimulated by thyroid-stimulating hormone from the anterior pituitary gland, which, in turn, is regulated by thyrotropin-releasing hormone from the hypothalamus. Once secreted, thyroid hormones circulate in the blood, primarily bound to proteins. However, only the unbound free fractions are metabolically active.
The most common disorder of the equine thyroid gland is neoplasia or hyperplasia. Mildly enlarged or neoplastic thyroid glands may be found at postmortem in older horses that showed no outward clinical signs and had normal circulating thyroid concentrations while the horse was alive. Occasionally, thyroid tumours have been associated with hyperthyroidism. Clinical signs of hyperthyroidism include weight loss, tachycardia, tachypnoea, hyperactive behaviour, ravenous appetite, alopecia, and cachexia.
Serum thyroid hormone concentrations should be measured in horses with enlarged thyroid glands and accompanying clinical signs. Additional diagnostic investigations include ultrasonography and biopsy; a T3 suppression test and/or nuclear scintigraphy may also be indicated. The diet should be examined to ensure iodine intake is appropriate because too little can result in goitre.
If thyroid neoplasia is diagnosed, it is not necessary to remove the thyroid glands immediately if serum thyroid hormone concentrations are normal and the glands are not enlarged enough to compromise breathing or eating. However, if the horse is hyperthyroid or if the glands begin enlarging rapidly, they should be surgically removed and the horse given thyroid hormone replacement therapy. As an alternative to surgery, propylthiouracil can be administered orally to decrease concentrations of circulating thyroid hormones.
The prevalence of primary hypothyroidism in adult horses is very low. Reported clinical signs of hypothyroidism include lethargy, exercise intolerance and poor coat hair. Hypothyroidism should be treated with thyroxine supplementation.
The adrenal cortices produce a variety of steroid hormones, including mineralocorticoids, glucocorticoids and adrenal androgens. These hormones are involved a wide range of physiologic roles electrolyte and fluid balance; cardiovascular homeostasis; carbohydrate, protein, and lipid metabolism; immune and inflammatory responses; and sexual development and reproductive function.
Adrenocortical dysfunction can manifest as either abnormal increases or decreases in activity. Primary hyperadrenocorticism and permanent hypoadrenocorticism (Addison’s disease) are rare in horses. However, hyperadrenocorticism may occur secondary to PPID and transient reversible adrenocortical dysfunction resulting in cortisol insufficiency can develop during critical illness.
Our understanding of hypothalamic-pituitary-adrenal (HPA) axis function in critically ill horses and foals is limited, but several studies suggest that critical illness-related corticosteroid insufficiency (CIRCI) may indeed occur in septic neonatal foals.
One case report of transient HPA axis dysfunction, as evidenced by both a low basal cortisol concentration and an impaired cortisol response to a high dose ACTH stimulation test, has been described in a septic neonatal foal3. In addition, two studies that measured basal ACTH and cortisol concentrations in healthy and septic neonatal foals found significantly increased ACTH:cortisol ratios in non-surviving septic foals4,5. Such high ACTH:cortisol ratios, with high ACTH concentrations and low corresponding cortisol concentrations, suggest HPA axis dysfunction at the level of the adrenal gland may occur in the septic foals.
HPA axis function has been further characterised in hospitalised foals using ACTH stimulation tests in two studies6,7. When human diagnostic criteria for CIRCI were adapted and applied to a group of 72 hospitalised foals, approximately 50% of all hospitalised foals and approximately 40% of septic foals met these criteria.
Furthermore, increased disease severity and poorer prognoses were correlated with decreased cortisol responses to ACTH stimulation in foals in both studies. Cortisol replacement therapy in the form of hydrocortisone at approximately 1mg/kg/d to 4mg/kg/d is recommended for the management of CIRCI in septic people and infants; however, such therapy has not been critically evaluated in neonatal foals to date.
Adrenocortical insufficiency is not well described in adult horses. Transient adrenal insufficiency characterised by low basal ACTH and cortisol concentrations, and impaired cortisol responses to ACTH stimulation testing has been described in one horse after abrupt cessation of long-term anabolic steroid supplementation.
Thus, the potential for iatrogenic adrenal insufficiency associated with HPA axis suppression by exogenous steroids should be considered in horses on chronic glucocorticoid or anabolic steroid supplementation, and care should be taken to avoid abrupt cessation of such therapy.