2 Jul 2018
Kirstie Pickles discusses some of the latest developments and studies into management and treatment for this condition.

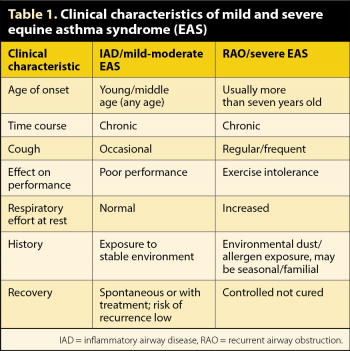
Equine asthma syndrome (EAS) has been defined by the American College of Veterinary Internal Medicine (ACVIM) consensus statement (Couëtil et al, 2016) as a spectrum of chronic inflammatory airway disease in horses that presents with chronic cough, excessive airway mucus and poor performance (Figure 1).

Within this spectrum, inflammatory airway disease (IAD) and recurrent airway obstruction (RAO) have been reclassified as mild and severe EAS, respectively. These are considered to be more appropriate than previous terms (IAD, RAO, heaves and chronic obstructive pulmonary disease) due to the many features these diseases have in common with each other and human asthma.
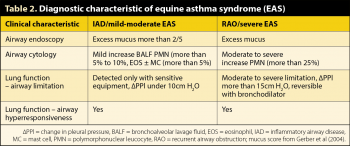
Although individuals with mild respiratory clinical signs have been reported to have an increased risk of developing RAO (Bosshard and Gerber, 2014), EAS is not considered to be a continuum. The majority of horses with mild EAS (IAD) recover and do not appear to develop severe EAS (RAO; Couëtil et al, 2016). Clinical and diagnostic features of EAS are summarised in Tables 1 and 2, respectively. A comprehensive review of severe EAS has been published (Pirie, 2014) and the ACVIM consensus statement (Couëtil et al, 2016) summarises knowledge of mild EAS.
EAS is considered to have a multifactorial aetiology, with both genetic and environmental factors appearing to be important. Despite considerable research, the understanding of the complex aetiopathogenesis of both mild and severe EAS is still incomplete.
The central role aerosolised allergens and endotoxin from hay and bedding play in the aetiology of severe EAS has been recognised for many years. Stabling is also reported to be a risk factor for mild EAS (Holcombe et al, 2001; Millerick-May et al, 2011; Ivester et al, 2014); however, the relative contribution of individual environmental factors is unknown.
The presence of high eosinophil and/or mast cell counts; and of Th-2 cytokines, such as IL-4 and IL-5, in the bronchoalveolar lavage fluid (BALF) of some horses with mild EAS supports a role for aeroallergens in this syndrome (Lavoie et al, 2011; Beekman et al, 2012). In both mild and severe EAS, once airway hyperreactivity is established, other non-allergenic components of the stable environment, such as particulates and ammonia, can exacerbate airway inflammation.
Human rhinovirus is an important trigger for the development and exacerbation of asthma (Busse et al, 2010). Interestingly, a greater percentage of horses with diagnosis of onset, or exacerbation, of mild EAS, defined by appropriate clinical history, BALF cytology and pulmonary function tests, had positive titres to equine rhinovirus A than control horses (Houtsma et al, 2015), leading to speculation of a similar role for equine viruses in EAS.
Additionally, a diagnosis of mild EAS was associated with nasal shedding of equine herpesvirus-2 (Houtsma et al, 2015). Although human asthmatics presenting with acute exacerbations do not have higher viral loads than non-asthmatics (Kennedy et al, 2014), it may be that a secondary event, such as from the environment, genetics, diet, age and/or immune response, precipitates a clinical episode. No conclusive evidence exists of a relationship between particular bacterial infections with mild EAS (Couëtil et al, 2016). Although an increased risk of mild EAS has been demonstrated in racehorses with increased number of bacterial colony-forming units per ml of tracheal wash (Wood et al, 2005), the causality of this association cannot be determined.
The genetics of EAS appear complex and further work is required in this area before the intricate genetics are elucidated. Offspring are 3.2 and 4.6 times more likely to develop severe EAS if one or both parents, respectively, are affected (Marti et al, 1991).
Horses with severe EAS are also more than seven times likely to have insect bite hypersensitivity (IBH) compared to control horses (Kehrli et al, 2015), suggesting a common immunogenetic background, route of sensitisation, or both. Furthermore, airway hyperreactivity and decreased partial pressure of arterial oxygen of a similar magnitude to that of mild EAS horses has been documented in horses with IBH without any overt clinical signs of respiratory disease (Lanz et al, 2017), suggesting that horses with IBH may be predisposed to developing EAS.
An association between severe EAS and the interleukin-4 receptor α-chain (IL4RA) gene has been documented in a high-prevalence severe EAS family (Jost et al, 2007). Additionally, a single nucleotide polymorphism (SNP), which is thought to be a regulatory mutation, has been reported on equine chromosome 13 near IL4RA in both this EAS family and unrelated severe EAS individuals (Shakhsi-Niaei et al, 2012). The IL4 receptor is involved in the regulation of IgE production and the stimulation of Th2-cells, and has been linked to human asthma and atopy (Ober et al, 2000; Youn et al, 2000).
A possible functional SNP in severe EAS has been identified in the protein-coding gene TXNDC11, which is suggested to play a role in regulating mucin expression and the production of hydrogen peroxide in the respiratory tract epithelium (Schnider et al, 2017).
Differential gene expression in paired endobronchial biopsies obtained pre-challenge and post-challenge from severely asthmatic and control horses revealed upregulation of gene sets involved in neutrophil chemotaxis, immune and inflammatory response, secretion, blood coagulation and apoptosis with MMP1, IL8, TLR4 and MMP9 appearing to be the most important proteins (Tessier et al, 2017).
The mainstay of management for severe EAS remains environmental dust management – controlling exacerbations with anti-inflammatories and bronchodilators. Mild EAS often responds to a similar treatment regimen.
The primary sources of stable aeroallergens are hay, straw and microbial growth in situ on deep litter bedding materials, such as wood shavings or newspaper (McGorum et al, 1998; Clements and Pirie, 2007a; b). Many owners seem unaware that deep litter beds, the position of muck heaps or hay/straw stores and content of neighbouring stables can significantly contribute to disease exacerbation. The lowest dust environment is outdoors; however, this is impractical for many horse owners.
Feedstuffs contribute the greater proportion of dust to the environment; especially dry hay (Clements and Pirie, 2007a), which is best replaced with haylage or steamed hay, if possible. Ground-fed hay decreases respirable dust in the horse’s breathing zone four-fold compared to a hay net (Ivester et al, 2012), while soaking hay decreases dust by 60% (Clements and Pirie, 2007b). Sensitive lung function tests have determined chronic subclinical peripheral airway obstruction can persist in severe EAS cases maintained in a low-dust environment, despite resolution of neutrophilic airway inflammation (Miskovic et al, 2007).
The effectiveness of oral, parenteral and inhaled corticosteroids in reducing clinical signs of severe EAS has been well documented (Cornelisse et al, 2004, Couëtil et al, 2006, DeLuca et al, 2008, Couroucé-Malblanc et al, 2008; Robinson et al, 2009; Grady et al, 2010); however, minimal information exists reporting their use in mild EAS.
A randomised cross-over trial of IM dexamethasone and inhaled fluticasone in horses with mild EAS reported equivalent, significant improvement in airway hypersensitivity and hyperreactivity with both treatments (Léguillette et al, 2017). Although no significant effect on clinical signs or BALF cytology was found, horses were maintained on dry hay throughout the study.
A dual inhalational and nebulisation device has opened up nebulisation as a practical route of drug delivery by horse owners. The nebulisation pump is very quiet and the mask is tolerated exceptionally well by horses. A proof of principle study using the device to nebulise dexamethasone to healthy horses confirmed good drug delivery to the lower airway with a median nebulised particle size of 3.5µm with 69% of particles under 5µm and 91% particles under 10µm (Haspel et al, 2018). Additionally, systemic bioavailability of dexamethasone was low (mean 4.3% ± 1.2%; range 0.5% to 8.2 %) with no effect on basal serum cortisol concentration or ACTH stimulation response.
Although healthy horses were used in this study, mean baseline percentage of neutrophils (13.5% ± 4.7%) was higher than that considered normal for control horses (Couëtil et al, 2016) and this decreased significantly (7.9% ± 3.5%) over the five-day nebulisation period, which was not significantly different to the response obtained by five days’ IV dexamethasone (Haspel et al, 2018). While this study appears to make nebulised dexamethasone an attractive treatment option for EAS, clinical efficacy and long-term safety studies are not available. Additionally, dexamethasone nebulisation to horses is an off-label use of the drug, which should, therefore, be appropriately considered and discussed with the client.
Inhaled and oral β2 agonist bronchodilators have been used for many years to improve corticosteroid deposition in the lung and in the treatment of acute severe EAS exacerbation. Down regulation of β2 adrenergic receptors occurs within 21 days (Read et al, 2012), although this can be prevented or reversed by coadministration of corticosteroids (Abraham et al, 2002).
As bronchospasm is largely mediated via muscarinic receptors, anticholinergic bronchodilators present a more rational therapeutic choice; however, drug delivery is less convenient with only IV or nebulised options. IV administration of N-butylscopolammonium bromide has been demonstrated to cause rapid onset of bronchodilation (Couëtil et al, 2012) and may be a safer choice than atropine for rescue therapy of acute severe EAS crisis due to its more selective mechanism of action and, therefore, lower risk of adverse side effects, such as decreased gut motility.
Despite widespread empirical use of bronchodilators in the treatment of mild EAS, the degree of bronchoconstriction in mild EAS is subclinical at rest and has not been well documented at rest or during exercise. Bronchodilators may not, therefore, improve airway opening in mild EAS, although coughing may be reduced.
Omega-3 fatty acid supplementation appears a useful addition to environmental management of EAS. A prospective, randomised, controlled clinical trial supplementing mild and severe EAS horses with 1.5g/day docosahexaenoic acid for two months, in addition to a low dust diet, induced greater, and more rapid, improvement in clinical signs, particularly coughing, compared to a low dust diet only (Nogradi et al, 2015).
Curcumin, the principal active ingredient in turmeric, has a potent inhibitory effect on neutrophil migration, myeloperoxidase release and induces neutrophil apoptosis, but has low oral bioavailability and solubility.
A blind, randomised cross-over trial giving twice-daily inhalation of a modified highly soluble curcumin derivate or control excipient to five severe EAS horses kept in a dusty environment for seven days resulted in a significant decrease in BALF total nucleated cell count and myeloperoxidase activity (Sanderson et al, 2011). As curcumin targets neutrophil activity, it may also be beneficial in neutrophilic mild EAS horses, but use in mild EAS horses with raised BALF eosinophil or mast cell counts would appear to have little value.
Cytosine-phosphate-guanosine-oligodeoxynucleotides bound to gelatin nanoparticles have been used as an adjuvant in clinical immunotherapy trials of human asthmatics (Senti et al, 2009) and atopic dogs (Mueller et al, 2005). These molecules are present in bacterial and viral DNA, and stimulate the mammalian immune system, including via toll like receptor 9, which are present in macrophages, bronchial epithelial cells, capillary endothelial cells and neutrophil granulocytes in equine lungs.
A randomised, double-blind, placebo-controlled clinical trial of 24 horses with severe EAS, exposed to hay, given one nebulisation of gelatin-bound nanoparticulates every two days, five times, resulted in significant improvement in clinical parameters and lung function that was sustained for four weeks post-cessation of treatment (Klier et al, 2015).
A further study comparing nanoparticulate therapy, with or without two individually determined allergens in severe EAS horses, found no additional benefit of allergens (Klier et al, 2018). Such immunotherapy may have value in the future.