25 Jun 2018
Jacqueline Matthews reviews the types of endoparasites impacting on horse health, each one's proliferation, new diagnostic protocols and strategies to gain owner compliance.
Equine helminths have been controlled for more than half a century using broad-spectrum anthelmintics. Traditionally, these medicines have been applied regularly to all horses in groups using interval deworming protocols.
The prolonged, frequent use of anthelmintics in such programmes has resulted in widespread anthelmintic resistance in several important worm species. For this reason, programmes need to take a more evidence-based approach to worm control. This is because no new equine anthelmintics are being developed in the short-term to medium-term and, if resistance to all current compounds develops, no options will be left for effective control of some of the most important pathogens of horses.
Three types of endoparasite are pathogens of horses:
Nematodes are the most important group in terms of their prevalence and potential pathogenicity. The most common species infecting horses and other equids are the small strongyles (known as cyathostomins). These worms are very common in grazing horses worldwide, with prevalence levels often reported in excess of 90%.
The cyathostomins are a complex group of worms comprising around 50 species. In most horses, the majority of the cyathostomin burden comprises 5 to 10 common species. Horses of all ages can be infected with these parasites, but higher burdens tend to occur in individuals of one to five years of age.
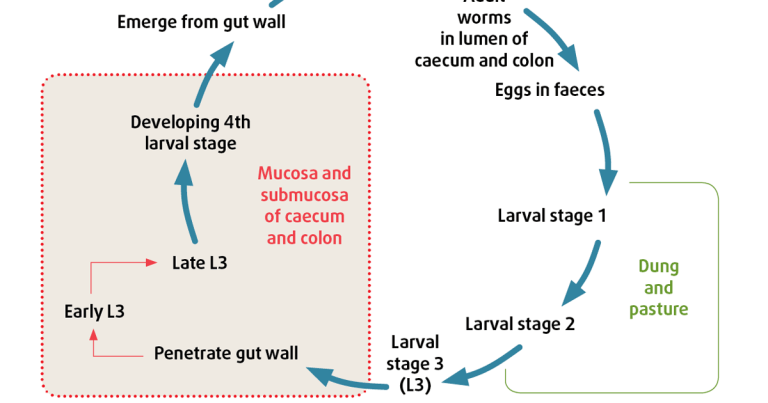
Cyathostomins have a typical strongylid life cycle (Figure 1). Worm eggs are deposited in dung. These hatch and develop through several pre-parasitic larval stages. Infective third-stage larvae migrate on to, and are ingested from, pasture. These stages can persist on pasture over winter from one year to the next. In the host, cyathostomin larvae can endure a prolonged phase in the intestinal wall, comprising several stages. These “encysted” larvae can persist for weeks to years and span the following stages:
The latter move from the intestinal wall to the lumen to develop to pre-adult, then adult, stages. Encysted larvae are important because they can cause severe pathology leading to colitis when they emerge from the gut wall in large numbers (several million larvae can build up in some individuals).
The EL3 stages are relatively refractory to most broad-spectrum anthelmintics, so must be targeted with specific treatments.
A main issue with cyathostomins is their ability to develop anthelmintic resistance. Resistance to benzimidazoles is almost ubiquitous in developed regions, with many cyathostomin populations that have been tested also identified as resistant to pyrantel compounds. Of concern, a shortened egg reappearance period (ERP) after anthelmintic treatment (a sign of emerging resistance) is being increasingly reported in cyathostomin populations after ivermectin or moxidectin treatment.
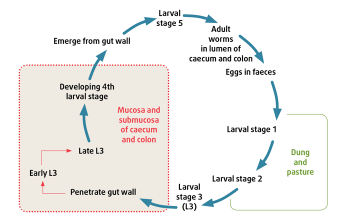
Strongylus vulgaris (large redworm) is the most important large strongyle species of horses. This parasite has a similar life cycle to the cyathostomins, apart from its migratory habits of larvae within the host. After ingestion, the larvae travel to, and develop in, the cranial mesenteric arteries (Figure 2). This worm is at low prevalence in the UK. This is likely due to the extensive application over the previous three decades of ivermectin and moxidectin, to which all stages of these worms are sensitive.
Damage caused by S vulgaris larvae in the cranial mesenteric arteries can lead to non-strangulating intestinal infarction and colic. This syndrome is observed in horses on poor deworming programmes, where animals have not received appropriate anthelmintic treatments and are grazed on contaminated permanent pastures.
Work in Denmark indicated the prevalence of S vulgaris infection has increased in the past decade. The authors of this work speculated this rise in prevalence was due to a sharp fall in the use of ivermectin and moxidectin because of the implementation of diagnostics-led deworming programmes in Denmark, which are now compulsory.
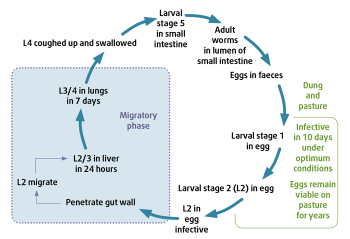
Parascaris equorum is an important nematode in foals and yearlings. It also has a migratory life cycle in the host (Figure 3), with hepatic and pulmonary stages. P equorum causes clinical signs when the infection load is high. Affected foals tend to be in poor condition, with respiratory signs. Foals can develop mild to severe colic, and P equorum-associated impaction colic has poor prognosis.
Similar to cyathostomins, anthelmintic resistance is a major issue in these worms, with resistance reported to the commonly used macrocyclic lactones ivermectin and moxidectin, as well as to pyrantel compounds. Anecdotal reports in the UK suggest low efficacy of all available broad-spectrum anthelmintics (such as fenbendazole, pyrantel embonate, ivermectin and moxidectin) in some populations.
Horses of all ages are susceptible to the equine pinworm, Oxyuris equi. Increasing reports of problems due to these worms have been reported, as well as possible lack of efficacy after treatment during the past decade. O equi spends most of its intra-host phase at the terminal end of the large intestine. After mating, female worms migrate to the perineal skin, where they lay eggs in yellow/grey gelatinous masses. The eggs develop to contain an infective third stage larva three to five days after laying. Horses are infected after ingesting larval-containing eggs.
O equi eggs are resistant to environmental conditions and can persist for prolonged periods. How long deposited eggs remain viable in the environment is unknown. Signs of infection vary from mild to intense pruritus in the region where eggs are laid. Many horses have pinworm infection without overt clinical signs.
The common tapeworm, Anoplocephala perfoliata, is usually seen in adult horses. Infection with these cestodes has been linked to impaction and spasmodic colic. The higher the burden of tapeworms, the more likely it is animals will develop clinical disease. Most horses harbour low tapeworm burdens, hence are unlikely to develop associated disease. A perfoliata has an indirect life cycle involving an oribatid mite intermediate host.
Tapeworms are hermaphrodite and fertilised eggs are found in worm segments that detach and then rupture, with eggs released intermittently in dung. Eggs are eaten by oribatid mites and horses are infected when they ingest mites containing infective larvae while grazing. Larvae attach to the gut wall and develop to adults at the small/large intestinal junction. Adult tapeworms can produce large numbers of eggs, which are excreted inconsistently.
The liver fluke, Fasciola hepatica, has been emerging as an issue in horses for the past couple of years. This parasite is a significant problem in the UK livestock industry, and has substantial impacts on sheep and cattle production. Horses, as well as humans, can become infected with this parasite should they be exposed to infective metacercarial cysts on contaminated pasture.
A rise in incidence in ruminants has been reported over the past decade; it is thought to be associated with changes in management practices, emerging anthelmintic resistance and changes in climate that are supportive of the development and survival of the pre-parasitic stags.
Liver fluke has an indirect life cycle, with involvement of a mud snail intermediate host. The snail prospers in damp areas (for example, marshland or river margins), so the presence of disease is associated with these types of environmental conditions. Metacercarial cysts are ingested from pasture and larvae hatch out, penetrate the gut wall and migrate through liver tissue, causing damage. Adult fluke live in the bile ducts and release eggs, which are excreted in dung. Clinical signs are usually vague in infected horses (for example, weight loss and increased serum liver enzymes) and the condition can be difficult to diagnose.
Other worms that can be encountered in equine practice include lungworm (Dictyocaulus arnfieldi) and threadworm (Strongyloides westeri). These worm species are far less common than the aforementioned and only observed in certain situations. Examples include when horses are grazed alongside donkeys (for instance, D arnfieldi) or in unhygienic conditions on stud farms (for example, S westeri).
For many years, strongyles were controlled by the administration of anthelmintics at regular intervals designed around the strongyle worm egg reappearance period (ERP) after treatment. The recommended timings were calculated from observations on worm egg reappearance times when the anthelmintic products were first licensed.
Although these programmes resulted in significant reductions in large strongyle-associated disease, they led to the development of widespread anthelmintic resistance in cyathostomin species, as well as in P equorum. For this reason, interval treatment approaches are not recommended. Programmes for strongyle control should comprise the following types of treatments:
1. Strategic group treatments designed to target important species or stages of worm that cannot be detected by standard parasitological diagnostic tests,
2. Targeted treatments to individuals with the decision to treat based on the outcome of a diagnostic test.
In the absence of specific diagnostic tests, strategic treatments must be applied to target strongyle larvae – in particular, cyathostomin larvae, which usually encyst in autumn/winter (in the UK).
At these times of year, the majority of the burden is not detectable by faecal egg count (FEC) testing, as most stages present are immature. Treatment with an anthelmintic with larvicidal activity (for instance, moxidectin) is recommended for all horses in late autumn or early winter for this purpose. This treatment will also target other worm stages undetectable by FEC testing, notably large strongyle larvae.
Note a second “winter” moxidectin treatment may be necessary (12 to 14 weeks after the first treatment). Application of this treatment should be based on the results of FEC testing, combined with a risk assessment of the likely levels of contamination on pasture. For example, young horses (below three years) are more likely to require such a treatment if they are grazing outside during a mild winter, especially if they are grazed at a high stocking density on permanent pastures not subjected to regular pasture hygiene measures. A blood test is in progress that shows promise for detecting cyathostomin infection. This is being developed to a commercial assay to help inform the application of anthelmintic treatments against encysted/luminal stages of these worms.
The main test used to inform the application of anthelmintics in horses is the FEC test. Treating horses at certain times of year (spring and summer) based on individual egg shedding can contribute greatly to reducing anthelmintic use, because worm egg excretion is over-dispersed, which means only a relatively small proportion of horses (usually 20% or fewer) have high levels of shedding (commonly referred to as the 20:80 rule). Hence, a sub-group of horses are responsible for the majority of contamination on to pasture, with the remainder having negligible or low egg shedding, and so only a small proportion require treatment.
Although it has taken time for FEC testing to become routinely used in practice, owners are increasingly using these tests to guide their treatment decisions. FEC testing should be performed from spring to early autumn in the UK. The decision to treat is based on a threshold of eggs per gram (EPG) detected in dung samples. By only treating horses shedding above the threshold, pasture contamination is reduced, but unnecessary treatments are avoided and selection pressure for anthelmintic resistance is reduced. Information on how to get the best value out of equine FEC tests is detailed later.
Control of P equorum is complicated due to the possibility of resistance to several classes of anthelmintic, in particular, macrocyclic lactones, which have the widest spectrum of activity against the different developmental stages of P equorum.
The anthelmintic programme applied should be based on a vet’s risk assessment of likely worm contamination levels, as well as the history of the anthelmintic products used on site. Because Parascaris migrating larvae cannot be detected by standard FEC tests, it is recommended anthelmintics be administered to foals at two to three months of age and at five to six months of age (the prepatent period of this parasite is approximately 10 weeks).
Options for treatment at these stages are fenbendazole, pyrantel or ivermectin, but keep in mind resistance to the latter compound is increasingly reported. For this reason, efficacy tests should be applied later in the season to check effectiveness of the products used against P equorum at each site.
After these first two “strategic” treatments, FEC tests can be performed once foals are seven or eight months old to establish the type of worm eggs they are excreting (that is, strongyle versus P equorum). If strongyle eggs alone are detected then foals should be treated with a macrocyclic lactone product. If P equorum eggs alone are detected, foals should be administered with a benzimidazole product. If both strongyle and P equorum eggs are detected, foals should be treated with pyrantel.
If it has been established the resident P equorum population is macrocyclic lactone sensitive, ivermectin can be used in this case. Ideally, as indicated previously, an efficacy test should be performed after treatment. If foals are seven to eight months old by late autumn/early winter, all individuals should be treated with moxidectin at this time.
An efficacy test should be undertaken to ensure the product has been successful in reducing strongyle and P equorum eggs. Because of the persistence of P equorum eggs in the environment, excellent pasture management (including regular dung removal) should be prioritised on foal pastures, and the same pasture should not be used for grazing foals year after year.
Anthelmintics licensed for A perfoliata treatment in the UK are praziquantel and pyrantel embonate (at twice the dose used for control of nematode infections). Unless otherwise specified (for example, when applying a strategic or FEC-informed application for nematode infection at the same time), anthelmintic products with a narrow spectrum should be used for tapeworm treatments.
This will become an issue because of the imminent removal of the only praziquantel product from the UK market. Because these worms are also over-dispersed in equine populations, treatments should be administered on the basis of diagnostic testing. Specific tests for tapeworm infection are outlined later in this article.
Horses should be treated for liver fluke only if infection is detected or strongly suspected. All flukicides must be prescribed under the cascade by a vet (www.gov.uk/guidance/the-cascade-prescribing-unauthorised-medicines), as no anti-liver fluke products are licensed for use in equids in the UK.
Triclabendazole is the anthelmintic of choice. This is due to its wider spectrum of activity against different fluke stages and a lower toxicity profile compared to other flukicides. A number of reports exist of triclabendazole resistance in liver fluke in sheep, so it is a possibility F hepatica populations horses are exposed to are resistant to this compound. Therefore, post-treatment monitoring for fluke eggs in dung (around six weeks after treatment) and observation for reductions in clinical signs and serum liver enzyme levels should be undertaken.
Where triclabendazole is shown to be ineffective, closantel may be considered. This is only effective against adult fluke, so a second application is required 8 to 10 weeks after the first treatment to target larval stages that had matured in the intervening period. Care should be taken with the dose administered.
Symptoms of overdose include blindness, anorexia and ataxia. Control must address the exclusion of snail habitats by fencing wet areas or improving drainage, to help break the parasite’s life cycle.
FEC tests only give an indication of worm egg excretion by individuals. FEC tests do not provide a measure of total worm burden. The test detects eggs released by female worms and does not take into account the number of adult male worms or developing larvae that can be abundant at certain times of year.
Standard flotation tests, such as the modified McMaster test, are best used for detecting nematode (strongyle and Parascaris) eggs. Fluke eggs are too heavy to be detected in these tests and also have poor sensitivity for cestode eggs. All horses in a group should be sampled and tested. Horses with an FEC result above a certain threshold (for instance, 200 to 500 EPG) are administered with anthelmintic. The EPG threshold that is selected depends on the yard’s management practices.
Lower thresholds should be chosen for groups of horses that are intensively grazed and/or have high proportions of young horses (that is, one to five years) present and/or where the grazing is not subject to dung removal. Sample timing depends on the last anthelmintic applied and its standard ERP. Thus, if the last product administered was moxidectin, a minimum of 13 weeks (the expected ERP) since the last treatment is recommended before sampling. For ivermectin, the minimum interval is eight weeks, and it is five to six weeks for pyrantel embonate or fenbendazole.
Collection, storage and handling of samples can affect the output of the test. For example, if eggs hatch, an underestimate will occur, so samples must be collected fresh or, at most, from dung excreted in the preceding 12 hours. Hatching requires an aerobic environment and temperatures above 6°C, so samples should be placed in sealable storage bags with all of the air expelled and stored below 6°C (especially if they do not reach the laboratory within a few hours). Samples kept below 6°C can be stored for up to five days before counting, but, after this time, egg numbers drop quickly. Ideally, samples should be counted as soon as possible after collection to reduce variability.
It is imperative a representative sample is collected from each dung pile. This is because worm eggs are not evenly distributed across dung and non-representative sampling will have a substantial impact on the accuracy of the count. To avoid this, at least three faecal balls should be collected at random from the heap or, if the balls are not obvious, multiple small sub-samples taken. The total amount sampled should be 30g to 40g.
Once at the laboratory, the material must be thoroughly mixed (ideally using a homogeniser) before any sub-samples are taken for egg counting. This substantially improves the accuracy of the test, as the material read is a more precise representation of the entire voided motion.
Several methods can be used to count nematode eggs and these vary in their diagnostic sensitivity, the time taken to perform them and the kit required to carry them out. When sensitivity is low (for example, the standard modified McMaster test, which uses a multiplication factor of 50 or 100 to obtain the estimated EPG), the test is more likely to give false-negative results at low FEC. Furthermore, large multiplication factors artificially inflate variance, leading to more varied results.
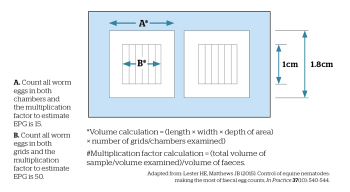
For these reasons, a test with either low or no multiplication factor to estimate EPG should be used. A method with a detection limit of 15 EPG or lower, especially when assessing anthelmintic efficacy, should be employed. With this in mind, the sensitivity of the modified McMaster method can be improved by counting a larger volume of the salt solution in the chamber. In the standard format, 3g of sample is mixed with 42ml flotation fluid and the suspension filtered through a sieve into a beaker.
An aliquot of this is pipetted into a McMaster slide, where the number of eggs counted in each of two grids is multiplied by 50 to give an estimated EPG. In this method, the detection limit depends on the volume of salt suspension examined, so the sensitivity of the test can be increased by counting all eggs in both chambers (Figure 3). Here, 1ml suspension is counted to reduce the detection limit to 15 EPG, thus increasing sensitivity and reducing variance. This can be done using a high-quality McMaster slide, which has an accurate chamber volume. If two slides are assessed per sample it will further increase test sensitivity; however, the increase in precision obtained is likely to be offset by the increased effort required.
FEC tests are useful in calculating anthelmintic efficacy. These should be performed once a year to test effectiveness of the products used, especially if pyrantel is being administered. In FEC reduction tests (FECRT), FEC tests are performed on egg-positive horses on the day of treatment and the EPG counted compared to matching samples taken from the same horses around two weeks later. Dung should be handled as previously described for the standard FEC test.
Because of variation in equine FEC, with many animals having a low FEC, it is important to test as many horses as possible. For this reason, a sensitive FEC method should be applied. Ideally, the test should be performed on horses that have a day-zero FEC of at least 200 EPG. These horses should have an accurate anthelmintic dose administered (based on bodyweight calculated using weigh scales or a calibrated girth tape). Follow-up samples should be collected in the same manner 14 to 17 days later. Once the second batch of counts is complete, the percentage FEC reduction can be calculated as follows:
Percentage of FEC reduction (FECR) =
([mean day 0 FEC – mean day 14 FEC] / mean day 0 FEC)
× 100
Thresholds for efficacy for the different licensed compounds are as follows:
If the mean group FECR is lower than these thresholds, first check it is not the case one or two horses have high FEC after treatment; they may not have received the full dose or spat out anthelmintic. Retest these individuals. In the UK, where resistance is suspected, it should be reported to the VMD’s Suspected Adverse Reaction Surveillance Scheme (https://www.gov.uk/report-veterinary-medicine-problem).
Standard FEC tests are not recommended detecting infection with A perfoliata, as cestode egg shedding is intermittent. A blood test is available that measures serum antibody specific to A perfoliata proteins (www.liverpool.ac.uk/diagnosteq/diagnostic-tests/tapeworm-antibody-test). A saliva test is also available (www.equisal.co.uk). Both tests are accurate in detecting tapeworm infections in horses.
Along with appropriate information on clinical and treatment history, the results from these tests can be used to inform on the need to apply anti-tapeworm treatments. The tests are best applied twice a year (for example, in spring and autumn). Horses can be tested for tapeworm infection from six months of age.
Pinworm can be detected using the “tape test”. Here, adhesive tape is used to take an impression from skin (not the hair) in the perineal area where female worms lay eggs. The tape is examined under the microscope for the presence of typical pinworm eggs (oval yellow eggs, with a “hatch” at one end).
Fluke eggs are not detectable by standard faecal flotation tests because these eggs are heavier than nematode eggs. For this reason, faecal sedimentation methods are required to identify patent infection. Several samples may need to be tested before fluke eggs are detected. A serum-based diagnostic test is available at the University of Liverpool (www.liverpool.ac.uk/testapet/test).
It is important to carry out good pasture management, such as regular dung removal (for instance, twice weekly). Dung must be completely removed from grazing and the adjacent areas. Field rotation and the resting of paddocks for six to nine months (for example, from autumn until the following summer) will substantially reduce the burden of parasites on pasture.
Achieving owner or yard compliance can be challenging and attained by highlighting the many advantages of an evidence-based worm control programme, these being:
Horse owners may think evidence-based programmes are more costly due to the need to obtain and analyse faecal, saliva and/or serum samples. However, data from a 2013 UK study using information from 16 yards (368 horses) demonstrated such programmes could be cheaper than interval ones. In this study, horses had FEC tests performed three times a year (between March and October) with a 200 EPG threshold applied for treatment.
All horses received moxidectin/praziquantel in winter to target strongyle larvae and tapeworms. Anthelmintic use was reduced by 82% compared to the interval programme (two moxidectin and two moxidectin/praziquantel treatments per horse per year) used by most yards just prior to the study. It was calculated, on the basis of costs at the time, an average saving of nearly £295 per yard per year was achieved in the evidence-based programme compared to the interval one.
With these criteria in mind, vets should engage with their clients to promote evidence-based control programmes and tailor each programme to the circumstances on each yard based on risk assessment of likely levels of contamination combined with diagnostic evidence of parasite load and anthelmintic resistance.
The author thanks The Horse Trust, the Horserace Betting Levy Board, The Thoroughbred Breeders Association and The Donkey Sanctuary for support of the studies described in this article.