4 Feb 2019
Jonathon Dixon offers diagnostic advice to first opinion practitioners, including when to refer, imaging and MRI benefits.

Figure 1. Transverse T1W gradient echo (GRE) and T2W fast spin echo images of the foot. The white arrows indicate a focal deep digital flexor tendon lesion and the red arrows indicate effusion of the navicular bursa.
Forelimb lameness in horses is common, and when assessing this the saying “no foot, no horse” often stands true, as the most frequently implicated regions would be the foot and associated distal limb regions.
To understand any subsequent findings, firstly, it is important to obtain a thorough clinical history; this should include the duration of lameness, description of the onset and any notable signs the owner identified. Further to this, notation of recent farriery interventions, and any previous treatments the owner has undertaken, is also vital.
Lameness assessment should then be performed. Initially, when assessing for forelimb lameness, we examine for the presence or absence of wounds, identify any overt issues with the limb conformation and foot balance, and identify the shoeing type or absence of shoes.
Appraisal of foot shape and size is important, as with forelimb lameness contraction of the lame limb hoof capsule with chronic lameness may be an issue . Gait analysis is then performed at the walk, trot and potentially (depending on extent of lameness) canter. Extensive lameness assessment is avoided in severely lame horses. It is useful to assess the gait on both a firm and soft surface, if available, and both a straight line and on the lunge. For some forelimb lameness, the gait abnormality may only be identifiable under saddle. In the author’s opinion, flexion tests and gait analysis systems can be useful as ancillary aids.
Following assessment, application of hoof testers is recommended, most importantly in cases with increased digital pulses and those with heat in the foot, as this may yield critical information. Careful palpation, including comparison to the contralateral limb, can localise a region of pain or abnormality, which could then be followed by either targeted imaging or, to confirm the site of lameness, ideally, diagnostic anaesthesia.
It is important to remember, with a few exceptions, the presence of a radiographic abnormality does not necessarily indicate the finding would be associated with pain and lameness. Use of diagnostic anaesthesia is, therefore, recommended for the clinician to be convinced of the region causing the lameness. This is performed via nerve blocking and the more specific the block placed, the more focused a clinician can be in follow-up testing. An example where this is clinically relevant is use of an abaxial sesamoid nerve block to assess for “foot lameness”. In reality, placement of this block will very likely desensitise the entire hoof capsule including the palmar heel region, pastern and, very likely, a significant portion of the fetlock; as such, this is not at all specific for “foot pain”. This is especially relevant given the potential for local anaesthetic diffusion (Nagy et al, 2009).
Diagnostic anaesthesia can be divided into perineural block placement or intrasynovial anaesthesia.
For the distal limb, the blocks commonly performed include:
Additionally, to be even more specific, the perineural anaesthesia can be performed either uniaxially or biaxially, and small volumes of local anaesthetic used.
Once the clinical examination has been undertaken, following diagnostic anaesthesia and the region associated with lameness has been localised, further investigations can be performed.
The distal limb has a number of vital structures that may be examined – namely, the DIP joint and associated collateral ligaments, navicular bone, deep digital flexor tendon, navicular bursa, collateral cartilages of the foot, collateral sesamoidean ligament, distal sesamoidean impar ligament and hoof capsule/laminae – to list a few. Lesions within these structures are common findings in forelimb-lame horses, and it is important to recognise that while some of these lesions may be identified radiographically, the soft tissue injuries and some bony lesions may simply not be visible.
The aforementioned region can be examined further in a number of ways, including radiographically, using ultrasound, or using an advanced imaging option, such as CT, nuclear scintigraphy (“bone scan”) or MRI. Radiographic examination forms the most frequently performed technique, with this allowing assessment of limb conformation, foot balance, joint congruity and for the presence or absence of periarticular bone change. An important limitation to recognise is articular cartilage cannot be radiographically appreciated without arthrography, therefore, the presence of either soft tissue injury or articular cartilage lesions, would not be identified.
Radiographs are appraised for image quality, initially, and that the structures of concern are included within the study, a minimum of two orthogonal projections of any region are always recommended, with more views being necessary in most cases to allow identification of relevant pathology. Bony structures should be assessed for abnormal shape, periarticular bone formation, abnormal alignment and abnormal radio-opacity. While detection of periarticular osteophytes (degenerative joint disease; DJD) is common, it is important to recognise the presence of one lesion does not imply the absence of another. A classic example would be the presence of DJD of the DIP joint in a horse with an acute onset lameness blocking to the navicular bursa or a palmar digital nerve block. This case may also have a significant soft tissue injury – that is, to the deep digital flexor tendon, which may be the cause of lameness – that is not radiographically visible.
If radiographs are appraised and no clear cause for the lameness is detected, or one is present that does not clinically “fit” with the presenting complaint, then soft tissue injury, or a bone oedema-like lesion, should be suspected and further investigations performed. At this stage, it is either prudent to consider referral for MRI, if financially possible for the client, or to pursue ultrasound examination of the regions accessible.
Ultrasound examination of the pastern and foot is realistically limited to the soft tissues proximal to, and through, the coronary band. This can allow for identification of the soft tissues of the pastern, the proximal portion of the DIP joint collateral ligaments and, with appropriate preparation, a transcuneal approach through the sole is possible to evaluate the insertional portion of the deep digital flexor tendon. The author does not frequently use this technique due to the availability and superior diagnostic capabilities of the advanced imaging options in the UK; however, some clinicians routinely use this. Ultrasonographic assessment of the soft tissues of the palmar pastern can include investigations of the flexor tendons, in the distal pastern in particular, and use of a microconvex transducer may complement what can be obtained using a traditional linear transducer.
Recently, ultrasound has also been used to describe a tendon-sparing approach to place a needle within the navicular bursa, which may be of clinical use.
The major benefits of MRI examination would be the ability to perform it in the standing sedated horse and the ability to assess, in high detail, both the soft tissues and the bony structures.
In the UK, MRI examination in standing horses is available at more than 20 institutions distributed throughout the country, resulting in many regions having access to a scanner without excessively lengthy transport. In addition, several equine hospitals with the ability to MRI horses under general anaesthesia, both with high and low-field units, are available.
In the most simplistic form, MRI works using the abundant presence of protons within biological tissue, a static magnetic field created by the magnet itself, and a radio frequency coil to transmit and receive information from the patient. As long as the coil can be physically placed around the region of interest and the patient fits within the magnet, high-quality images can be obtained.
The most frequently imaged region in standing horses is the feet and with respect to the standing MRI scanner in use in the UK, the field of view where images are obtained at any one time is a sphere in the region of 15cm in diameter. For this reason, an entire series of images would be obtained of the foot of most horses with this; however, this typically does not include the pastern or fetlock, and these areas must be scanned separately. The scans are most often performed standing, under the aid of sedation, and are completely non-invasive, which is beneficial.
The two most evident limitations or downsides of the modality performed in this way are that of patient-derived movement artefacts and the relatively lengthy time for image acquisition – roughly 45 to 60 minutes per foot. This being said, the use of MRI has completely revolutionised the ability to diagnose injuries to the distal limb of horses and is commonplace in the diagnostic arsenal of equine practitioners.
Images can be acquired in any orientation required, but typically in a minimum of three planes and a variety of pulse sequences are performed, typically including T1W, T2*W/T2W and fat suppressed (short TI inversion recovery; STIR) images. The type of scan performed dictates the overall appearance of the images and differing scan types allow for example identification of fluid presence within bone or for soft tissue pathology (deep digital flexor tendinopathy).
MRI should be recommended for the following:
The major benefits of MRI are it allows for identification of soft tissue lesions – for example, those affecting the deep digital flexor tendon within the hoof capsule, one of the most commonly injured structures. With identification comes the ability to classify the lesion into varying types (core, dorsal border, parasagittal split, insertional lesion) and the extent of the abnormal region. This also allows for identification of involvement of the adjacent structures, such as fibrillation of torn tendon fibres into the navicular bursa (Figure 1), or potential adhesion formation associated with torn tendon fibres to the collateral sesamoidean ligament or navicular bone.
Several published studies exist documenting the associations with both the presence of tendon injuries and outcome overall, which indicate a low proportion of horses with such injuries return to work, and more specific evidence regarding prognostic indicators. These include presence of deep digital flexor tendon lesions within the foot, that are longer than 30mm in length and have a cross-sectional area of more than 10 per cent being associated with a poor clinical outcome, and core lesions or parasagittal splits that were associated with an outcome often worse than those with dorsal border lesions (Cillán García et al, 2013).
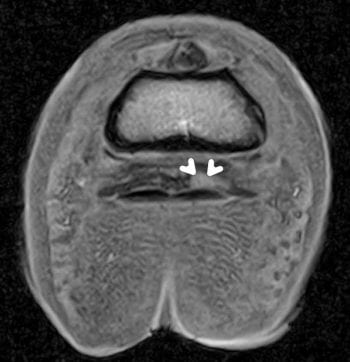
Presence of fluid distension of the navicular bursa (Figure 2), or soft tissue presence within this (granuloma formation), can often be detected, resulting in a possible different course of treatment. While some of these lesions may have been identifiable ultrasonographically, the use of MRI facilitates detailed examination of this region and would form one of the main benefits of performing this technique – ultimately providing useful information to the client.
As may be expected, the presence of concurrent navicular bone pathology negatively influences the outcome of horses with deep digital flexor tendinopathy. Navicular bone assessment can be challenging radiographically, with large anatomic variation, and challenges in interpretation of subtle changes.
Even in some horses with significant pathology identified on MRI, the radiographic examination may be unremarkable. MRI examination allows for assessment of all borders of the bone. In particular, focus is often given to the palmar (flexor) border, which opposes the deep digital flexor tendon (Figures 3a and 3b). Deep erosions may be present in this surface, which are very often associated with lameness, and there may be concurrent tendon pathology and adhesion formation. Likewise, abnormal signal patterns within the spongiosa of the navicular bone may be identified on MRI, which may be undetectable using any other technique – reportedly indicating fibroplasia, osteonecrosis, irregular trabeculae, oedema, haemorrhage, degenerate adipose tissue and capillary infiltration with enlarged intertrabecular bone spaces.
Thickening of the compact bone surrounding the structure can also occur, with other abnormalities including distal border fragments, enlarged synovial invaginations, proximal/distal elongation of the palmar compacta and fractures/multipartite navicular bone. Further examination also allows identification of lesions associated with the supporting ligaments, including the collateral sesamoidean ligament, the distal sesamoidean impar ligament and the chondro sesamoidean ligaments.
Assessment of the DIP joint collateral ligaments in their entirety is possible using MRI – this being a strong benefit – whereas assessment is highly limited using conventional techniques (such as ultrasound). Some technical features exist that affect how this structure is interpreted, and the main finding to be aware of is that of magic angle artefact (seen at 54.7°±10° to the static magnetic field and multiples thereafter). Overall, injuries to these structures may be characterised (Dyson et al, 2004) by abnormal signal patterns (typically increased signal on T2W and STIR fast spin echo; FSE images), loss of the normal clearly defined architecture and enlargement (Figure 4).
Periligamentous signal alterations may also occur, and associated bony lesions at the origin and insertion on to the phalanges (Figure 5). Increased signal as a solitary finding, without enlargement or bone lesions, should be cautiously interpreted in case of the confounding effects of magic angle artefact. Should significant lesions of the DIP joint collateral ligament be identified, including partial or complete rupture, then examination of the joint should be carefully performed, as articular damage and periarticular bone formation can often occur. Joint effusion and synovial proliferation can all be assessed in detail (Figure 6).
It is not uncommon for the collateral (ungular) cartilages of the foot to become mineralised, especially in heavy breed horses. A variable number of separate centres of ossification can be present, which, as a solitary finding, are not necessarily a significant abnormality. In a small number of horses, trauma at the base of the cartilage or a fracture may occur. These may not be visible radiographically and MRI may be required for identification. Presence of extensive fluid accumulation within the cartilage or ossified cartilage may be identified, which can represent oedema or haemorrhage, often in combination with some regional sclerosis. While these injuries may be visible scintigraphically, diagnosis usually requires MRI examination and the underlying aetiology is often unclear.
Distal phalanx lesions, which may not be radiographically identified, include osseous cyst-like lesions, insertional entheseopathy associated with the distal sesamoidean impar ligament or potentially distal phalanx fractures (Figure 7). The use of cross-sectional imaging facilitates assessment of fracture position and, in particular, can clarify the position of the fracture plane as either articular or non-articular. As would be expected, extensive surrounding signal changes often occur within the bone. In some cases of severe lameness, MRI can be used to identify the extent of more severe injuries, including in cases with previous foot penetration (Figure 8) or synovial sepsis and osteomyelitis (Figure 9).
A further specific benefit of MRI is the ability to suppress the signal associated with marrow fat and, as a result, identify fluid presence within bone. Bone oedema-like lesions can be caused by a variety of inciting factors; however, the presence of high signal on fat suppressed images (STIR FSE), in conjunction with identification of phase cancellation artefacts, can identify the location and extent of such change. In these cases radiographs may be expected to appear normal (Figure 10).
In summary, with the evolution of advanced imaging for the distal limb, the ability to accurately diagnose lameness in this region has improved exponentially. Further investigations will need to be performed to understand how this truly applies to improving outcomes and directing specific therapies, and it is hoped this will continue to be performed moving forward as ever-increasing case numbers are identified and horses are followed up over time.