12 Aug 2019
Jacqueline Matthews and Corrine Austin discuss managing and treating this parasite in horses, as well as integrating treatment in sustainable control programmes.

Image: Austin Davis Biologics
Gastrointestinal helminth infections are almost ubiquitous in grazing equids. These infections comprise a mixture of nematode and cestode infections, and animals that graze contaminated pasture that are not subject to effective control measures are at risk of life-threatening disease.
The most common helminths of horses are the small strongyles (cyathostomins) and tapeworms of the genus Anoplocephala.
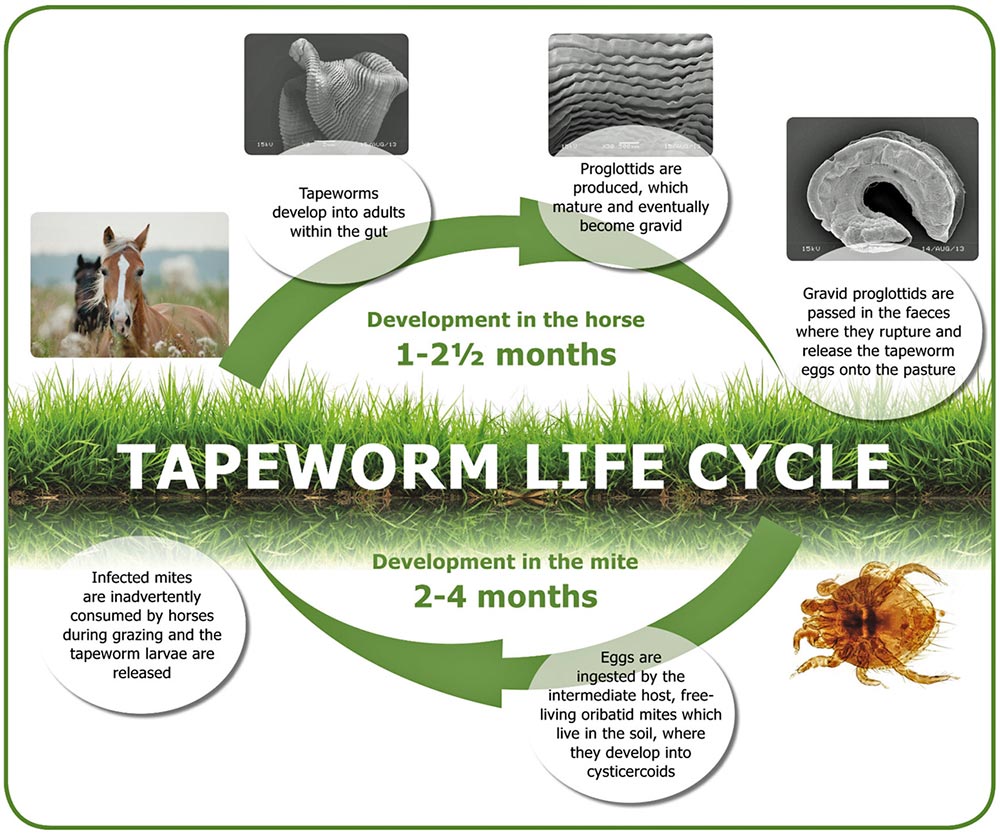
Anoplocephala perfoliata is the most common equine tapeworm species. It has an indirect life cycle requiring an intermediate oribatid mite host. Infected horses pass tapeworm eggs on to pasture, where they are consumed by mites (Figure 1). Eggs develop into larvae and remain in situ until mites are ingested by a horse, in which larvae are released into the intestine. The life cycle completes when larvae attach to the intestinal wall, where they develop into adults, which subsequently release eggs.
Oribatid mites live in grass and soil; the number of infected mites depends on the number of infected horses and their levels of infection. Infected mites represent a reservoir of infection, but it is unknown how long they can persist on pasture for. To reduce contamination, infected equids should be identified and treated with anthelmintics to reduce egg shedding into the environment.
Unlike small strongyle infections, anthelmintic resistance has yet to be reported in Anoplocephala species; however, resistance to praziquantel has been reported in the dog tapeworm, Dipylidium caninum, demonstrating the potential threat of drug resistance in cestode species1. The fact it is extremely difficult to establish infection levels soon after treatment means controlled studies to investigate anthelmintic efficacy against Anoplocephala species are almost impossible to perform.
The authors are aware of anecdotal field reports that suggest poor efficacy of anti-tapeworm treatments in equine populations in the UK. Such reports highlight the threat of resistance in Anoplocephala species and warrant caution because of the potential pathogenicity of these common parasites.
This threat is exacerbated by the fact restricted treatment options are available (that is, praziquantel or pyrantel at twice the dose recommended for anti-nematode treatments) to target these infections.
For this reason, practitioners need to apply more sustainable control strategies and exploit diagnostic tests that enable identification of individuals with worm burden levels above a set threshold for targeting treatments. Using more targeted strategies will reduce anthelmintic use and apply lower selection pressure for drug resistance in tapeworm populations.
Most healthy adult horses are not infected with tapeworm, or they carry a low burden with no signs of clinical disease. However, in some individuals, these parasites can build up in high numbers, leading to colic as a result of ileocaecal impaction, intestinal intussusception or rupture and subsequent peritonitis2,3.
Infection intensity has been shown to correlate with the severity of histopathological lesions affecting the ileocaecal mucosa and ganglionitis of the enteric plexus4,5.
Surveys in the UK and abroad have indicated many owners treat their horses far too frequently with anthelmintics. Progress has been made in the past decade in the UK and other parts of the EU, at least, with reports having shown increasing uptake of evidence-based control measures6,7, but, in the main, reports have indicated horses are often given anthelmintics without due consideration as to whether they actually need treatment.
With no new anti-tapeworm dewormer classes on the horizon, it is imperative to safeguard these medicines and stop indiscriminate treatments. For nematode infections, faecal egg count (FEC) analysis is used to target treatments to reduce egg shedding in individuals. This takes into account the over-dispersed nature of infections in horses such that, at any given time, only a small proportion of a population (often 20% or less) require treatment to reduce nematode egg shedding8.
Unfortunately, for tapeworm infections, coprological diagnosis is unreliable – in part due to the sporadic nature of egg shedding, as well as the poor distribution of eggs in faeces and a poor sensitivity of the tests available9.
Modified methods involving centrifugation and flotation have been developed that demonstrate sensitivity levels of up to 61%, but these are time-consuming and labour-intensive to perform10. Sensitivity of the centrifugation/flotation technique is higher (90%) if diagnosing infections of greater than 20 tapeworms11.
As previously indicated, FEC methods do not provide a good indicator of tapeworm infections; these have a relatively low sensitivity and egg shedding is intermittent. A serum-based ELISA accurately diagnoses infections by detecting tapeworm-specific IgG(T) in blood12 and has been commercially available for more than a decade. More recently, to facilitate uptake of testing, a non-invasive saliva IgG(T) antibody test was developed13, based on the same antigens used in the serum ELISA.
Commercial availability of the saliva-based test has revolutionised the way tapeworm infections are managed, and equine practitioners are increasingly using this test in health management programmes. The test, which accurately diagnoses horses with tapeworm infection, has been available in the UK and Europe for several years, and is proving invaluable to worm control plans in the face of the impending threat of anthelmintic resistance.
The saliva test was validated by comparing antigen-specific antibody levels in 104 equine saliva samples, with matching tapeworm burdens enumerated at postmortem. This study demonstrated the saliva test was able to differentiate horses with “low” burdens (0 tapeworm) from those with “moderate/high” burdens (greater than 1 tapeworm) with a sensitivity of 83% and specificity of 85%13. Horses misclassified as “low” in this validation study had a burden of no more than 20 tapeworms – and, therefore, no horses with tapeworm burdens higher than this threshold were misdiagnosed.
As it is considered the presence of 20 tapeworms or less is not clinically relevant14-16, this suggests the test correctly identified all pathogenic burdens, keeping in mind pathogenesis has been correlated with infection intensity5. Importantly, the saliva test shows similar sensitivity to modified FEC tests (90%) when used to diagnose higher (20-plus) burdens and has proven to have increased sensitivity when diagnosing low (1-plus) burdens; this may include prepatent infections that would not be identified using FEC methodologies.
When the test is used to inform on treatment options or as a differential diagnostic, horses are ascribed a “saliva score”, where those with a score of less than -0.09 are assigned as “low” and those with a score greater than 0.6 assigned as “moderate/high”. Anthelmintic treatment is recommended when a score greater than or equal to -0.09 is obtained.
As with any antibody test, antigen-specific Ig half-life following treatment is an important consideration when interpreting results. Research has suggested salivary antibodies are shorter-lived than antibodies in blood and, with respect to the saliva test, antigen-specific salivary antibodies in horses (n=11) drop below the treatment threshold within three months of treatment13. The shorter half-life can be rationalised by differences between mucosal and systemic immune responses.
Salivary tapeworm-specific IgG(T) is part of the mucosal response to infection and probably derived from plasma cells that originated as recirculating B-blasts triggered in the gut submucosa at the site of infection (Figure 2)17.
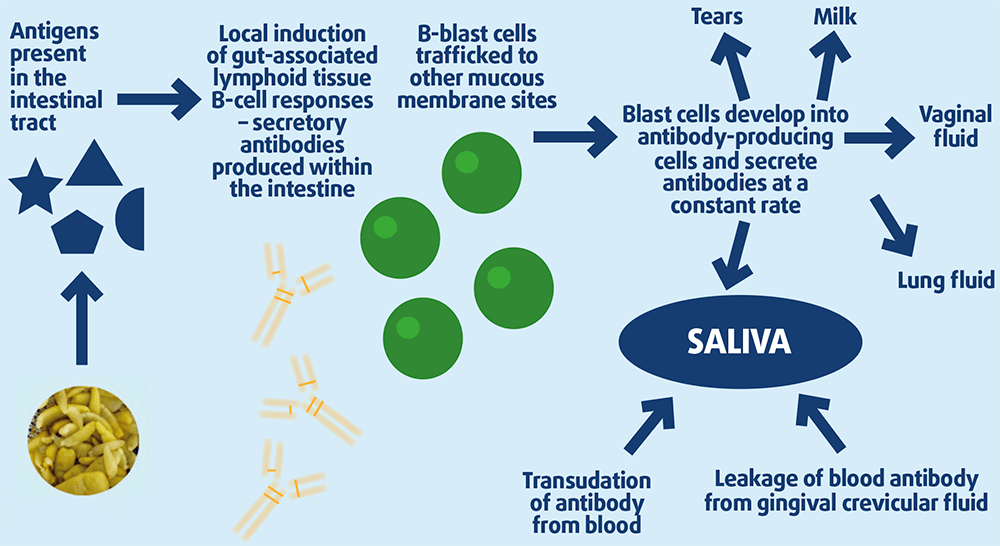
The dynamics of tapeworm-specific saliva antibodies will depend on an individual and, importantly, whether re-infection occurs. If testing is performed twice a year and/or at least four months after treatment, it is likely tapeworm-specific salivary antibodies – if detected – are due to current, rather than historic, infection.
Effective control plans must consider management, local climatic conditions and worm life cycles. It is impossible to eradicate all worms from all horses using anthelmintics, and trying to do so only selects for resistance. Therefore, control plans must seek to reduce environmental levels of infection using good management, combined with targeting particular worm types and/or stages with the right anthelmintic to prevent disease.
As most adult horses kept under good management have low burdens, this distribution enables the use of diagnostic-led protocols, leading to substantially reduced anthelmintic use. By using specific tests to inform the treatment needs of individuals, only those horses that genuinely require therapy receive anthelmintic. The advantage of using such tests means horses at risk of higher burdens – and, hence, potential disease – are identified.
The authors’ study18 that tested saliva from welfare sanctuary horses (n=237) using the saliva test showed that, in autumn, 85% of the horses tested were below the treatment threshold. Indeed, the majority (71%) of these remained below the treatment threshold in three further tests in the following 12 months.
Of the 69 horses positive in the first test, and that received treatment, 7 required treatment following three subsequent tests, while greater than 50% of horses administered with anthelmintic fell below the treatment threshold at the following test. The data suggested no increase in tapeworm prevalence in the 237 horses during the study period, despite a considerable reduction (86%) in anthelmintic usage compared to a standard “blanket” treatment programme.
Targeted control protocols also need to incorporate good pasture management; it is recommended dung be removed from pasture at least twice-weekly. In addition, fields should not be overgrazed and rested where possible.
Alternate/mixed grazing with ruminants can be practised as most helminths are host species-specific, but potential infection with Fasciola hepatica (liver fluke) must be considered – especially when sheep are allowed to graze the same pasture. Harrowing is not recommended unless pastures are rested for a sufficient time to allow significant worm mortality.
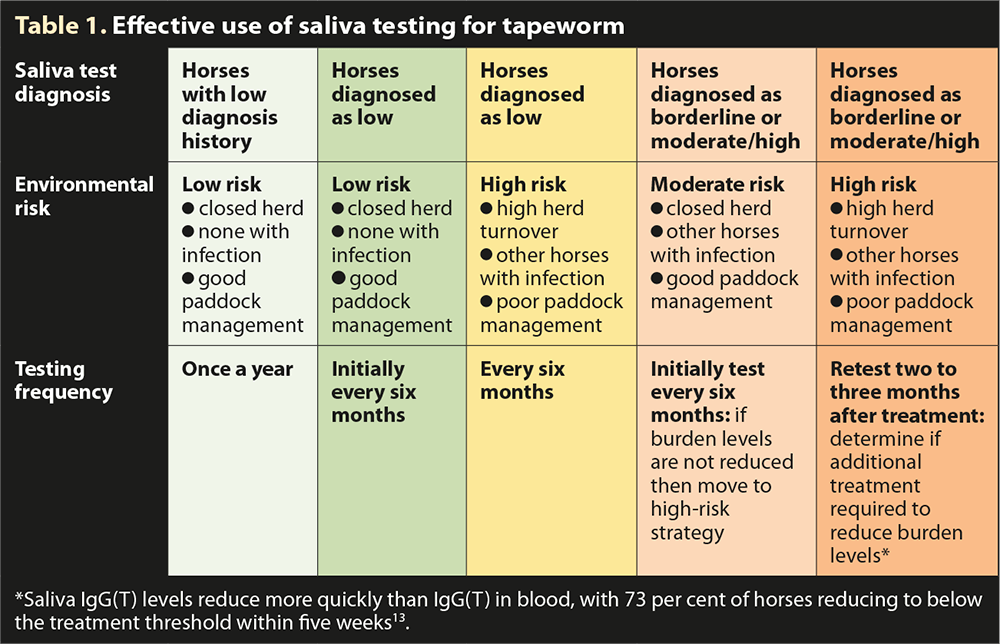
In all cases, a risk assessment of likely parasite transmission should be undertaken, taking account of the animals present, the type of grazing management, historic use of anthelmintics, clinical history and any data generated from diagnostic tests. Each yard is unique and the plan needs refining for each situation. In the case of tapeworm control, it is recommended horses be tested every 6 to 12 months depending on the individual’s risk (Table 1). If an annual test is conducted, this is best scheduled in autumn.
A study that investigated Anoplocephala species prevalence in Denmark (greater than 11,000 observations) over seven years (2009-15) showed egg shedding prevalence was highest in autumn19; however, seasonality was not very marked, with the data demonstrating Anoplocephala egg shedding in all seasons. It is worth noting that, in this large study, Anoplocephala prevalence was highest in younger horses (one to five years old) and risk of infection was not associated with soil type.
With a positive diagnosis for tapeworm and no treatment requirement for strongyle or ascarid infections, treatment options are pyrantel (at twice the dose recommended for anti-nematode treatments) or a praziquantel-only formulation. In the UK, the latter option is now only available as a specials medicine, which can be dispensed by a veterinary surgeon under the prescribing cascade.
In the UK, specials medicine manufacturing is closely regulated by the VMD and enables vets to treat animals using drugs not licensed for a specific condition in that species.
In cases where anti-nematode treatment is indicated, depending on the time of year, combination productions can be used, such as those containing praziquantel and ivermectin (in summer/spring based on evidence of nematode egg shedding detected by FEC) or praziquantel and moxidectin (in autumn/winter, to target encysted cyathostomin larvae and large strongyle larvae).
The use of diagnostic tests to predict equine tapeworm burden and inform the application of treatments will reduce the frequency of anti-cestode anthelmintic use in practice. This hypothetically will reduce selection pressure for resistance – not just in tapeworm, but in nematodes, too, as commonly used products contain a combination of praziquantel and macrocyclic lactones.
Resistance to macrocyclic lactones and pyrantel in nematodes (in particular, in small strongyle species) is a major concern. Although anthelmintic resistance has yet to be documented in A perfoliata and other tapeworm species, it is not unforeseeable that, with the widespread application of regular blanket treatments, resistance is inevitable.
The saliva-based tapeworm test provides a solution to reduce treatment frequency in practice and help protect efficacy of anthelmintics for the future.