4 Dec 2017
Rachel Agass and Barny Fraser describe conditions affecting joints in horses, while also considering various diagnostic and treatment methods.

Lameness and musculoskeletal injuries are common in the equine patient. Joint pain and inflammation accounts for a high proportion of lameness encountered in all types of horses.
An exhaustive description of joint pathology is beyond the scope of this article; however, some general principles and broad categories of disease are discussed.
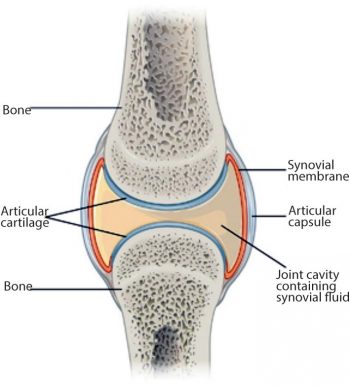
Joints or articulations are formed by the union of two or more bones, with the uniting medium chiefly cartilage and fibrous tissue. Three chief subdivisions of joints are recognised: synarthroses, amphiarthroses and diarthroses.
Synarthroses are immovable unions, including the sutures of the skull, and the pelvic and mandibular symphyses. Amphiarthroses confer a small amount of movement, and include the sacroiliac and tarsometatarsal joints. This discussion will focus mainly on diarthroses – moveable or “true“ joints. Diarthrodial joints are made up of several components, including articular (hyaline) cartilage, joint capsule (including the synovial membrane and the external fibrous capsule), synovial fluid and subchondral bone, as well as any articular menisci and periarticular ligaments (Figure 1).
Osteoarthritic joints are characterised by loss of, and damage to, articular cartilage matrix components, resulting in decreased joint function (Frisbie, 2012). The inciting cause in OA may either be primary degeneration (wear and tear) resulting from excessive forces on normal cartilage, or normal forces on already defective cartilage (for example, osteochondrosis). The sequence of events in naturally occurring disease is more variable, but may involve damage to one or more of any of the components of the joint, which has the potential to initiate a perpetuating cycle of degenerative change within the synovial cavity (McIlwraith, 2002). Most equine joint pathology is likely to be induced by acute trauma, or repetitive loading or overloading (Pool and Meagher, 1990).

Inflammation (synovitis) occurs through release of inflammatory mediators, such as cytokines – principally interleukin (IL)-1 and tumour necrosis factor-alpha (TNFα) – and prostaglandins, which, in turn, alter the balance between tissue anabolism and catabolism, ultimately resulting in cartilage degradation. Matrix metalloproteinases (MMPs) play a role in the metabolism of normal joints. Tissue inhibitors of metalloproteinase (TIMPs) bind to MMPs to form inactive complexes. In an osteoarthritic joint, the balance between MMPs and TIMPs alters, favouring a catabolic state.
Another large group of joint pathologies falls under the umbrella term “developmental orthopaedic disease” – generally referred to as osteochondrosis and including osteochondritis dissecans (OCD). This is a multifactorial disease process that involves focal failure of endochondral ossification within the articular epiphyseal or metaphyseal growth cartilage (Ekman et al, 2009). The exact aetiology remains unclear; however, evidence suggests a hereditary component (Ytrehus et al, 2007), although other potential factors have been implicated, including higher growth rates, over-nutrition, trauma, stress or mineral imbalances (copper), but evidence for these factors remains equivocal (Ytrehus et al, 2004; Gee et al, 2007).
In osteochondrosis, biomechanical loading is thought to result in focal microvascular changes that lead to ischaemia, retention of cartilage cores and subsequent chondronecrosis, resulting in the formation of an osteochondral flap, which has the potential to detach and become a free-floating fragment within the joint (McIlwraith, 2011).
Osseous cyst-like lesions may develop as a result of continued biomechanical loading on retained chondronecrotic lesions in juvenile animals (Denoix et al, 2013; McIlwraith, 2011); however, other aetiologies, including trauma, have been suggested.
Additionally, synovial sepsis is a relatively common equine condition, generally occurring either as a result of a wound penetrating a synovial cavity, or through haematogenous infection – most commonly observed in foals with transient or persistent bacteraemia. Contamination of the otherwise sterile synovial environment and bacterial colonisation leads to a marked acute synovitis, resulting from the release of pro-inflammatory mediators – especially IL-1 and TNFα.
Clinical signs of joint disease include lameness, swelling or effusion (Figure 2), as well as pain on palpation or flexion and reduced range of motion. Clinical examination remains the most important diagnostic tool and does not require expensive equipment to perform. Subtle effusions can be detected through palpation and range of motion, and the presence of pain on palpation are important considerations.
Clinical examination can often (of course with exception) provide important information regarding localising clinical signs and guide further tests (diagnostic imaging, synoviocentesis and diagnostic anaesthesia) to reach a diagnosis.
Where lameness is present, positive response to intrasynovial anaesthesia provides a good indication for diagnostic imaging. Care must be taken to interpret the response to diagnostic anaesthesia, as false positive and false negative results can, and do, occur. For example, intrasynovial anaesthesia of the distal interphalangeal joint often desensitises the navicular bone, bursa and associated soft tissues, as well as much of the sole. Conversely, intrasynovial fetlock anaesthesia may not alleviate pain associated with subchondral bone in palmar/plantar osteochondral disease, owing to the neuroanatomy of the subchondral bone of the distal third metacarpal/tarsal bone (Pilsworth and Dyson, 2015).
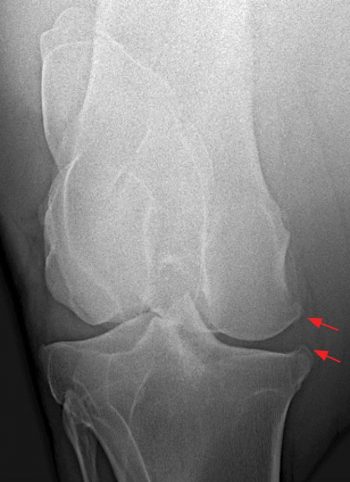
Results of diagnostic anaesthesia must, therefore, be interpreted in the light of physical examination and diagnostic imaging findings. Consideration should be given to the signalment history and degree of lameness prior to performing diagnostic anaesthesia. If the presence of an intra-articular fracture is possible, diagnostic imaging should be carried out prior to blocking to avoid the development of a catastrophic fracture (Figure 3).
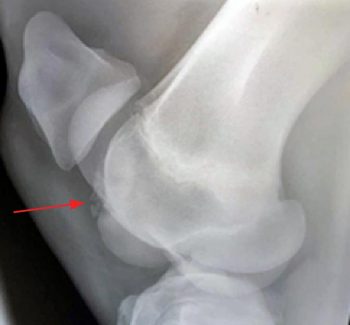
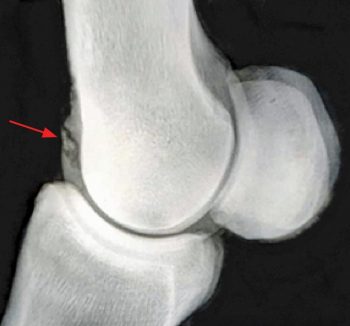
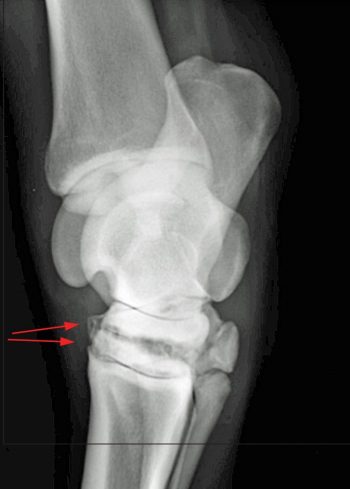
Radiography probably remains the most widely available and commonly used diagnostic imaging modality. Additional projections may be useful on a case-by-case basis and some of these are included, along with some of the pathologies these may identify. Radiographic signs of OA include presence of periarticular osteophytes, subchondral lysis and sclerosis, enthesophytes, lesions within the subchondral bone, as well as narrowing or widening of the joint space. The latter should be interpreted with caution and stressed views may be taken to confirm this. Figure 4 demonstrates examples of articular pathology.
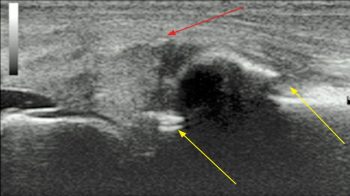
Ultrasound examination is very useful to assess joint disease (Figure 5). It allows visualisation of the periarticular soft tissues, the presence and character of joint effusions – which may be variably palpable – as well as the articular cartilage and bone surfaces. Linear 7.5 megahertz (MHz) to 12MHz ultrasound probes usually provide adequate diagnostic imaging of the majority of equine diarthrodial joints.
Curvilinear or microconvex probes may provide additional information regarding structures such as the cruciate ligaments. Dynamic and Doppler ultrasound exams of joints are now increasing the level of information gained from a basic ultrasound exam.
Nuclear scintigraphy is not the primary diagnostic imaging modality for assessment of OA in horses. It may, however, be appropriate for horses with multiple limb or shifting limb lameness, which may be more difficult subjects for diagnostic local anaesthesia, and where no obvious localising clinical signs exist. As with many other diagnostic tests, sensitivity and specificity are variable. Scintigraphic uptake in the distal tarsal region can be used to strengthen a diagnosis of OA, since its sensitivity compared to MRI for distal tarsal OA has been shown to be excellent (Daniel et al, 2012). Examples of significant scintigraphy findings are seen in Figure 6.
MRI and CT are becoming increasingly available in referral settings and both have their place in diagnosing OA. MRI can offer the advantage of standing imaging, particularly useful for feet and distal limbs, and CT provides a cost-effective and efficient means of image acquisition with which bone and soft tissue – particularly with contrast enhancement – can be thoroughly evaluated. These modalities can provide more current physiologic information, rather than the traditional historic picture seen with radiography alone.
Broadly speaking, treatment can be divided into conservative and surgical methods.
Osteoarthritic treatments can be divided into symptom-modifying and disease-modifying anti-osteoarthritic drugs (SMOAD and DMOAD, respectively). Treatments exerting SMOAD effects purely help alleviate clinical signs, whereas treatments with DMOAD effects can alter the disease process.
NSAIDs are probably the most widely used systemic analgesics for many equine conditions, and OA is no exception. Several licensed equine products are available, including phenylbutazone, flunixin meglumine, meloxicam, ketoprofen, firocoxib and suxibuzone. Labelled doses are often for once-daily administration, but are commonly given twice daily to provide adequate analgesia.
Concerns regarding side effects (gastrointestinal – ulceration and right dorsal colitis – and renal) mean these drugs are perhaps best used conservatively in at-risk patients. High drug dosages for prolonged periods and combinations of NSAIDs administered concurrently should be avoided to reduce the risk of adverse effects.
In general, NSAIDs are relatively inexpensive, easily administered and often effective at reducing the level of lameness associated with OA. Their use is often not applicable to competing animals, where long withhold periods are necessary prior to participation, although meloxicam has a 72-hour withhold after multiple doses.
Topical diclofenac has been shown to have some SMOAD effects in experimentally induced middle carpal arthritis, in which treated limbs demonstrate increased articular glycosaminoglycan (GAG) contents, as well as significantly less radial carpal bone sclerosis and overall cartilage erosion compared to untreated limbs (Frisbie et al, 2007).
Tiludronic acid is licensed for the treatment of spavin and clodronic acid is licensed for the treatment of navicular disease. Additional common uses for these products include impinging dorsal spinous processes and third carpal bone sclerosis, among others.
The mechanism of action involves inhibition of osteoclasts and decreasing secretion of IL-6 (a potent osteoclast inhibitor) from osteoblasts (Tokuda et al, 1998). Additionally, some direct anti-inflammatory effects appear to exist.
Evidence for efficacy in horses includes the prevention of bone mineral density loss in treated horses with distal limb casts.
Horses with OA of the distal tarsal joints experienced improved grades of lameness when treated with tiludronic acid versus controls. Tiludronic acid is administered as an IV infusion in 1L of saline, whereas clodronic acid is administered via the IM route, at three separate sites.
Developed in the southern hemisphere and available in the UK as a feed supplement, scientific evidence supports the beneficial effects of a plant oil extract in the treatment and prevention of OA.
Studies have demonstrated reduced prostaglandin E2 concentrations in synovial fluid of treated horses following osteochondral fragment removal, compared to horses treated with surgery alone (Pearson et al, 2013) and the product has an excellent safety profile. Evidence has also suggested it results in improved chondrocyte viability and proliferation in cartilage explants exposed to IL-1 (Pearson et al, 2008).
The product is formulated as a gel or granules and is only available for client use through a veterinary practice. In addition to the scientific evidence for efficacy, anecdotal reports are promising; in a study of evidence base for glucosamine-based nutraceuticals, research supporting its efficacy came out top (Pearson and Lindinger, 2009).
Large numbers of oral joint supplements are available that mainly aim to provide the building blocks of molecules integral to articular cartilage – usually including chondroitin sulphate, glucosamine, hyaluronan or a combination of all three.
Chondroitin and glucosamine seem to have good oral bioavailability in the horse (Du et al, 2004). However, the link between oral absorption and clinically therapeutic levels is yet to be determined.
Human studies have indicated efficacy. Anecdotal reports are often favourable in horses, but only poorly controlled equine clinical studies have been performed.
A further concern is, as these are uncontrolled, often a major discrepancy exists between the contents of the packet and what is claimed on the label.
Corticosteroids have potent anti-inflammatory actions and have, therefore, been a mainstay of treatment for OA for many years.
Pain relief is largely through inhibition of prostaglandin release as a result of inhibiting the enzymes phospholipase A2 and cyclooxygenase-2 expression in the arachidonic acid cascade (Caron, 2005). Additionally, triamcinolone acetonide (TA) has disease-modification properties, acting as potent inhibitors of cytokines (IL-1 and TNFα) responsible for ongoing inflammation and pathogenic articular cartilage degeneration (Amano et al, 1993; Caron, 2005).
Low doses of triamcinolone demonstrate cartilage-sparing effects, through decreased production of degradative enzymes (MMPs; Williams and Brandt, 1985), although repeated administration of high doses in vigorously exercised horses may affect proteoglycan synthesis unfavourably (Todhunter et al, 1996).
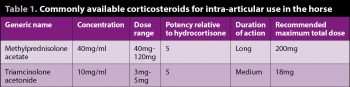
TA and methylprednisolone acetate (MPA) are the two most readily available products in the UK and are licensed for intra-articular use. Dexamethasone can also be administered in synovial structures, although it lacks a licence for this purpose.
Table 1 details products and recommended maximal doses. It is noteworthy a growing number of clinicians are decreasing the doses of corticosteroid used – where, traditionally, a dose of 10mg TA would be used per joint treated, doses of 3mg to 5mg are more commonly applied, with the same therapeutic effect. Informed consent should be sought prior to treatment, particularly with respect to the risk of synovial sepsis and laminitis.
Evidence suggests MPA (especially in combination with lidocaine) may exhibit chondrotoxic effects in a bovine model of synovitis (Seshadri et al, 2009), whereas TA seems to exert a chondroprotective effect (Frisbie et al, 1997); therefore, MPA is often reserved for use in lower motion joints, such as the distal tarsal joints.
The conventional theory has been no need exists to worry about the state of the articular cartilage in these joints and MPA might be able to promote ankylosis. As yet, no evidence suggests ankylosis can be promoted in this fashion (McIlwraith, 2010).
It has been suggested concurrent administration of intra-articular hyaluronan (HA) may mitigate the negative effects of MPA, but firm evidence for this theory is lacking.
Also, no direct evidence supports the combination of TA and HA; however, HA-treated OA joints have been observed to have less significant cartilage fibrillation. The combination, therefore, does have logic and is favoured by some.
Both corticosteroids and HA have the drawback of reducing the number of bacteria required to promote an infection and, as such, should be administered under strict asepsis. The addition of an aminoglycoside antibiotic is contraindicated due to the binding affect and inactivation of exogenous HA.
Regenerative medicines can be broadly divided into two categories – the non-expanded and expanded products. Non-expanded therapies include platelet-rich plasma (PRP) and autologous conditioned serum (ACS)/IL-1 receptor antagonist protein (IRAP). These products are subject to a more rapid turnaround, as cell populations are not increased, therefore are available for use shortly after collection (24 hours for IRAP).
Evidence for efficacy in equines is increasing, but based on human studies treating naturally occurring OA; however, they are used with significant frequency by some clinicians. One equine study that examined the intra-articular use of ACS suggested significant decrease in the degree of lameness at 70 days after surgical induction of synovitis in IRAP-treated horses (on days 14, 21, 28 and 35) compared to saline controls (Frisbie et al, 2007).
A small study investigated the intra-articular use of PRP in five horses injected three times at two-week intervals. The horses demonstrated improvements in the degree of lameness and joint effusion – most obvious at two months, and persisting for eight months. A systematic review of equine and human use of PRP concluded: “Although the majority of equine clinical studies yielded positive results, the human clinical trials failed to corroborate these findings,“ and: “The use of PRP in musculoskeletal lesions, although safe and promising, has still not shown strong evidence in clinical scenarios“ (Brossi et al, 2015).

Expanded products are those in which the cell populations are processed post-collection, via cell isolation and amplification methods over a period of two to four weeks. This means a delay between collection and treatment of the horse. Mesenchymal stem cells (MSCs) are such an example, commonly collected from bone marrow of the sternebrae of iliac crest, although adipose-derived stem cells collected from the SC tissue have also been used.
Bone marrow-derived MSCs have demonstrated chondrogenic potential superior to that of adipose-derived MSCs (Vidal et al, 2008). However, in vivo studies are generally lacking for intra-articular use. One study described an experimental model of OA in goats, in which the cranial cruciate ligament was transected and the medial meniscus damaged. Bone marrow-derived MSCs were injected intra-articularly, suspended in hyaluronic acid three weeks following injury.
Treated joints demonstrated reduced cartilage destruction, osteophyte formation and subchondral bone sclerosis versus controls, and meniscal restoration was noted at six weeks, suggesting a potential therapeutic use for MSCs in OA (Murphy et al, 2003). Stem cells have been used in horses with stifle injuries following arthroscopic surgery, with some indications of favourable outcome, particularly in more severely affected joints (Ferris et al, 2014).
Polyacrylamide hydrogel consists of 97.5% water and 2.5% cross-linked polyacrylamide. The molecule is homogeneous, biocompatible, non-degradable and does not migrate, but integrates with host tissues. Since the hydrogel is non-absorbable, it is suggested to provide augmentation lasting years.
Though in equine terms its use is in its infancy, it has been used for more than a decade in human medicine, with an excellent safety profile. Its mechanism of action is thought to be due to a physical cushioning effect, and it is administered at 1ml to 2ml per joint, depending on the joint size. Initial results are encouraging, with up to 82.5% of treated horses being non-lame at 24-month follow-up, with joint effusion also decreasing over time (Tnibar et al, 2015), when a single fetlock or carpal joint was affected.

No adverse effects have been recorded. Its effects are reported to be incremental, with a steady improvement in lameness over eight months after a single injection.
Numerous indications for arthroscopic surgery exist for diagnostic or treatment purposes. However, an exhaustive list will not be described here. One common indication for surgical management is for the treatment of OCD. OCD is a dynamic process in horses younger than 12 months, and some lesions heal naturally; some tarsocrural lesions have been shown to resolve radiographically in foals aged up to 5 months and some femoropatellar lesions up to the age of 8 to 12 months (Dik et al, 1999). Beyond this time, lesions tend to persist.
Those lesions associated with lameness or significant effusion are unlikely to heal without intervention and, in these cases, arthroscopic debridement is indicated. Additionally, many clinically silent lesions are surgically managed for sales purposes – for example, prior to Thoroughbred yearling sales.
Removal of OCD fragments in other breeds, in the absence of clinical signs, is debatable, and often the decision is based on owner and/or surgeon preference. Arthroscopy can be used simultaneously as a method of diagnosis and treatment when radiographic findings are inconclusive.
Another indication for surgical intervention includes selected cases of traumatic injury. This may be for the management of synovial sepsis or for debridement of intra-articular soft tissues, damage to which may result in fibre prolapse and, therefore, drive synovitis and the development of OA. Additionally, many intra-articular fractures are amenable to arthroscopic surgery, which may facilitate fragment removal or fracture fixation, depending on the nature of the fracture. Other indications include debridement of articular cartilage lesions, with or without stimulated endogenous cartilage repair.
In summary, the main aims of treatment in equine joint disease are to control the intra-articular inflammation to reduce cartilage damage and degeneration. Often, a combination of treatments is superior to one treatment modality alone.