12 Oct 2021
Camilla Scott BVetMed, CertAVP(ESM), DipACT, MRCVS, discusses transition management, the oestrous cycle, oestrous synchronisation and induction of ovulation.

Image: matilda553 / Adobe Stock
Increased pressure to breed mares outside of their physiological breeding season, limited availability of semen from competing stallions, pressure to breed a large number of mares to a given stallion and the desire to obtain multiple pregnancies from embryo donors has resulted in hormone manipulation in mares being commonplace.
Due to a commercial emphasis being placed on early born foals, breeders are wanting to cover/inseminate mares when there is a seasonal absence of follicular development. Various protocols have been tried and tested for advancing the first ovulation of the year; the most common method is providing an artificial photoperiod; however, alternative or concurrent therapeutic intervention may be required.
Indications for oestrus synchronisation in the mare include lining up donor and recipient mares for embryo transfer, programming maiden and barren mares to be bred at the beginning of the season, and timing AI of mares with shipped semen. Methods include termination of the luteal phase with exogenous prostaglandins, lengthening the luteal phase often with exogenous progestins such as altrenogest, induction of ovulation with luteinising hormone and gonadotropin-releasing hormone analogues, and inhibition of the follicular phase.
Increased pressure to breed mares outside of the physiological breeding season, limited availability of semen from competing stallions, pressure to breed a large number of mares to a given stallion and the desire to obtain multiple pregnancies from embryo donors has resulted in hormone manipulation in mares being commonplace.
The mare is a seasonally polyoestrous long-day breeder with a physiological breeding season lasting from April to October in the northern hemisphere. The hypothalamic-pituitary-gonadal axis in the mare is subject to a circannual endogenous rhythm that is primarily regulated by day length. Other factors such as environmental conditions, nutrition, age and breed have also been shown to influence seasonal reproduction in the mare.
Increasing ambient photoperiod in the spring reduces the duration of melatonin secretion, resulting in stimulation of hypothalamic gonadotropin-releasing hormone (GnRH) secretion, which in turn triggers pituitary follicle-stimulating hormone release and subsequent follicular growth (Ginther, 1992). Initially follicles are steriodogenically incompetent, resulting in waves of follicle growth and regression, and irregular or prolonged oestrous behaviour.
Positive feedback on luteinising hormone (LH) secretion only occurs when a dominant follicle becomes steriodiogenically competent and is able to release sufficient oestrogen to cause an LH surge, resulting in the first ovulation of the year and the end of the transition period.
Exposure of mares in deep anoestrus to a stimulatory photoperiod remains the most successful method of advancing the first ovulation of the season. It is important to realise that an artificial photoperiod doesn’t shorten transition, but simply initiates it sooner. The duration from onset of adequate light exposure to ovulation is approximately 60 days, although considerable variation exists between individuals.
The most commonly used lighting regimen is providing a fixed length of 15 to 16 hours of light exposure and 8 to 9 hours of dark, with a minimum light intensity in a stable of 100 lux (100W to 200W bulb). Other methods include using an additional 2.5 hours of light beginning at sunset and a pulse lighting system, providing 1 hour of light, 9.5 to 10.5 hours after the onset of darkness, during the photosensitive phase (reviewed by McCue et al, 2007).
Alternatively, the Equilume light masks provide a unilateral LED light source emitting 50 lux of blue light directly to the eye during the four hours after dusk.
Many hormone protocols have been studied, with variable results. Therapy is typically more effective when started either in late transitional mares or following a period of stimulatory artificial photoperiod.
Dopamine receptor antagonists sulpiride and domperidone have been shown to increase circulating levels of prolactin, and stimulate follicular growth and/or ovulation during spring transition (Panzani et al, 2011). The exact mechanism of action of dopamine in the mare is unclear, but it is thought to act directly on the ovary through the D2 receptor protein to inhibit follicular growth, hence the stimulatory effect of an antagonist.
It is also hypothesised that hastening of dopamine antagonist-treated mares is due in large part to increased sensitivity of the follicle to LH through greater receptor numbers following elevated prolactin levels. The results are, however, variable between studies and individual mares.
A minimum follicular size of 25mm if required before treatment is likely to be successful, and if no effect is seen after 10 days it is unlikely to be successful thereafter and another treatment protocol should be initiated.
Progestins such as altrenogest and progesterone in oil have been shown to be ineffective in mares in deep anoestrus, and have no consistent effect on follicular development and gonadotropin secretion patterns in mares in early transition. Progestins will, however, suppress erratic oestrous cycles during transition and synchronise the first ovulation of the year, in mares in mid-to late transition and those exposed previously to an artificial photoperiod.
Progesterone treatment in conjunction with oestrogen may be a more reliable method to programme the first ovulation of the season. The addition of oestrogen results in a more uniform suppression of follicular growth and, hence, improved synchronisation once treatment is discontinued.
Vaginal inserts such as Cue-Mare and progesterone-releasing intravaginal devices have been shown to be very effective in late transitional mares, with 95.2% of treated mares being served within the first 21 days of the season compared to 42.6% of controls in a study by Hanlon and Firth (2012).
Mares should be examined for follicular growth at 7 and 10 days following insertion. Once a dominant follicle (greater than or equal to 35mm diameter) is identified, the vaginal insert is removed and the mare monitored closely until she is in oestrus, at which stage an ovulation induction agent is given and either natural cover or AI is performed 24 to 36 hours later.
Native GnRH and GnRH agonists such as buserelin, deslorelin, gonadorelin and goserelin have been used to increase gonadotropin release – and, hence, follicular growth and ovulation. Ovulation rate and number of treatment days have been shown to correlate to follicular size at the onset of treatment and depth of anoestrus. Mares that develop a preovulatory follicle should receive human chorionic gonadotropin (hCG) as an ovulatory induction agent. Mares in deep anoestrus are at risk of returning to anoestrus following a GnRH‑induced ovulation, whereas those in transition are more likely to continue to cycle.
Recombinant follicle-stimulating hormone (FSH), purified equine FSH and equine pituitary extract have been shown experimentally to stimulate follicular growth and ovulation in mares deep in anoestrus. With the addition of recombinant equine (re), LH mares that did not conceive continued to cycle on the reFSH/reLH protocol compared to those on reFSH alone, which returned to anoestrus (Loud et al, 2014). Unfortunately, these products are not currently commercially available.
The mean duration of the oestrous cycle is 21 days in mares and 23 days in ponies, but varies dependent on the time of year, reproductive status and age of the mare, with longer cycles seen in the spring months in non-lactating and older mares. The length of the oestrous cycle is dictated by a set period of dioestrus of 14 to 15 days and a variable oestrous period usually lasting between 3 to 7 days.
Oestrus, also known as the follicular phase, is the period of the oestrous cycle when the mare is sexually receptive to the stallion, stimulated by secretion of oestradiol from growing follicles and the absence of progesterone due to the lack of a corpus luteum (CL). The variable duration of oestrus is dependent on the size of the follicles present at the end of dioestrus, the growth rate of the follicles during oestrus and the final size of the dominant follicle at ovulation.
Mares typically have one to two follicular waves, with the major follicular wave usually emerging seven to eight days post-ovulation, consisting of a cohort of 7 to 11 follicles of approximately 5mm, which enter a six to seven-day growth phase, growing by 3mm/day to 5mm/day.
The dominant follicle usually has a 2mm to 3mm size advantage over the other follicles, often developing a day earlier than the other follicles in the same cohort. Deviation occurs at the end of the common growth phase once the dominant follicle reaches a size of 20mm to 25mm; during this phase the dominant follicle has increased FSH sensitivity, increased oestradiol production, increased levels of free insulin-like growth factor-I and increased specificity to respond to LH through induction of granulosa cell LH receptors.
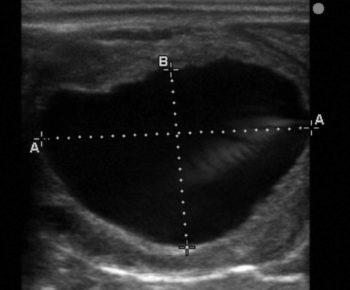
Production of inhibin and oestradiol by the larger dominant follicle(s) results in a decline in FSH levels, and regression of smaller non-dominant follicles, as they are not sensitive to low circulating FSH and lack LH receptors. LH stimulation of the dominant follicle causes further follicular growth with the preovulatory follicle reaching an average size of 40mm. Ovulation typically then occurs approximately 24 to 48 hours prior to the end of oestrus (Figure 1).
The incidence of spontaneous double ovulations in the mare is reported between approximately 2% in ponies and 25% in thoroughbreds. Follicular growth of two dominant follicles is typically slower than that of one, resulting in a smaller preovulatory follicle diameter in twin ovulating mares. Ovulations may occur synchronously (within 12 hours) or at intervals of two days or more.
Dioestrus or the luteal phase is characterised by a period where the mare is under the influence of progesterone secreted by the CL following ovulation of a dominant follicle. During this time the mare will be unresponsive to the stallion. Dioestrus in the non-pregnant mare lasts between 14 to 15 days.
Progesterone levels rapidly rise following ovulation from 1ng/ml to 2ng/ml to peak levels of up to 8ng/ml 16ng/ml on day 8 before slowly decreasing until the onset of luteolysis. In the absence of maternal recognition of pregnancy, luteolysis occurs between 14 to 16 days post-ovulation due to endometrial secretion of prostaglandin F2α (PGF2α) stimulated by oxytocin production. Luteolysis results in a return to basal progesterone levels and signs of behavioural oestrus.
Transrectal palpation of a mare in oestrus will reveal a soft relaxed cervix, a heavy oedematous uterus, and the presence of large and softening ovarian follicles. Ultrasound examination will reveal a characteristic oedema pattern of the uterus in response to oestrogen secretion and large fluid‑filled hypoechoic follicles.
In contrast, mares in dioestrus will have a tight cervix and a toned uterus on palpation with a homogenous pattern, and no evidence of uterine oedema on ultrasound examination. A corpus haemorrhagicum or CL will be present on ovarian ultrasound.
Oestrous synchronisation in the mare can be unreliable due to both a variable length of oestrus (three to seven days) and an unreliable time of ovulation. While 69% of mares ovulate in the final 48 hours of oestrus, 14% ovulate after the end of oestrus, and hence synchronisation for a single breeding time is difficult.
The most common indication for oestrous synchronisation is during embryo transfer when the recipient mare is synchronised to ovulate one day before to four days after the donor mare. Synchronisation may also be used to programme maiden and barren mares so that they can be bred at the beginning of the season.
Methods of oestrous synchronisation include termination of the luteal phase, lengthening of the luteal phase, induction of ovulation and inhibition of the follicular phase.
Exogenous PGF2α (250μg cloprostenol IM or 5mg to 10mg dinoprost IM) is widely used to terminate the luteal phase (“short‑cycling”) by causing lysis of the CL and return to oestrus. A single injection will only, however, be effective if the CL is at least five days old, as prior to this luteal tissue is still forming and the CL doesn’t have sufficient prostaglandin F receptors to respond. Serial injections (10mg dinoprost IM twice a day for three days) from as early as 24 hours following ovulation has been shown to prevent CL formation in appropriately 60% of mares (Rubio et al, 2008).
Following treatment with PGF2α, mares will typically return to oestrus between two and five days later. Ovulation is, however, highly variable dependent on the stage of development of the follicles at the time of prostaglandin administration. For example, follicles with a diameter of less than 10mm may take up to 10 days before ovulating, whereas follicles of approximately 25mm diameter may take three to four days to ovulate and follicles greater than 40mm may ovulate or luteinise straight away.
Exogenous progestins such as altrenogest can be used to lengthen the luteal phase. LH release from the anterior pituitary is inhibited, so ovulation is unlikely to occur; however, no effect exists on folliculogenesis in the mid-luteal phase with follicular growth continuing. Recommended protocols often suggest a 10-day course of altrenogest with an injection of PGF2α on the final day to lyse any previous luteal tissue that may be present.
The combination of progesterone and oestrogen causes a more profound negative feedback than progesterone alone, resulting in a more uniform inhibition of follicular development so that when treatment is discontinued ovulation can be more closely synchronised, with 70% of mares ovulating between 10 to 12 days of the final day of treatment.
Hormonal induction of ovulation is commonly used to coordinate the timing of breeding/insemination closely to that of ovulation. In addition to improvements in fertility, ovulation induction minimises the number of times a mare needs to be bred/inseminated during the oestrous cycle. This has significant advantages when semen availability is limited, but also in mares prone to breeding-induced endometritis.
When a mare is in oestrus with a relaxed cervix, uterine oedema and a follicle of at least 35mm diameter, ovulation induction agents can be used to more reliably predict the timing of ovulation. The most commonly used and commercially available drugs are hCG and GnRH analogues (commonly deslorelin).
hCG (1,500IU IV) is a large glycoprotein extracted from the urine of pregnant women, which has an LH-like effect in the mare resulting in ovulation approximately 36 hours (36 to 48 hours) following administration. Repeated use, high doses and IM administration has been associated with antibody formation, so repeated use is not recommended within the same breeding season. Older mares may also show reduced sensitivity to hCG.
GnRH analogues (deslorelin acetate 1.5mg IM injection and deslorelin 2.1mg biodegradable short-term SC implant) stimulate the release of both FSH, resulting in follicular growth and LH resulting in ovulation approximately 40 hours (38 to 42 hours) post-administration.
Advantages over hCG include a more predictable response, the ability to be used repeatedly within the same season and tightening the window of multiple ovulations, therefore improving subsequent twin reductions or multiple embryo flushes.
Disadvantages include increased inter‑ovulatory periods associated with SC implants due to temporary downregulation of pituitary gonadotropin secretion and suppression of ovarian follicular development (Johnson et al, 2002). This can, however, be avoided by placing the implants in the vulva mucosa and removing them post-ovulation or by using the injectable form, deslorelin acetate.
Mares may display discomfort on ovarian palpation following a recent ovulation and collapse of the follicular wall can often be detected. Ultrasound examination will reveal lutenisation of the previous dominant follicle and the presence of either a blood/fibrin filled corpus haemorrhagicum with an echogenic rim and hypoechoic centre or a more homogenous echogenic CL.