17 Jul 2023

Skin cytology is an extremely quick, inexpensive and non-invasive tool to obtain relevant information in veterinary dermatology. It can be applied to sample several types of skin lesions, such as pustules, papules, scales, crusts, ulcers, nodules or plaques.
Cytology allows for differentiation between inflammatory and neoplastic lesions, and to discern between benign and malignant tumours. Moreover, it allows for visualising aetiologic agents, such as bacteria, yeast, fungi or protozoa.
Cytology can be performed with six different techniques: fine-needle with or without aspiration, glass slide apposition, adhesive tape apposition, skin scraping and swab sample.
Fine-needle aspiration is performed to sample nodules or plaques. After trichotomy and skin disinfection, a 2.5ml syringe-connected needle is inserted into the lesion. While moving the needle back and forth inside the lesion, negative pressure is applied by keeping the syringe in aspiration. Then, it is released and the needle is removed from the lesion.
After disconnection of the needle from the syringe, the latter is filled with air, the needle is replaced on to the syringe and the cells previously collected in the cone are expelled on to the slide.
Afterwards, a second slide is used to spread out the sample. The two slides are placed perpendicularly and slid in opposite directions to obtain a cell monolayer. The same technique can be used without aspiration, and this is preferred to sample skin lesions rich in blood.
Skin cytology can also be obtained directly through glass slide impression on to the skin. This technique is used to sample superficial lesions, such as crusts, pustules, epidermal collarettes, scales, erosions or ulcers.
The edge of the slide is usually used to lift crusts or epidermal collarettes, or to break pustules. This technique often aims to identify aetiologic agents, and consequently, the area should not be disinfected before sampling.
On exfoliative or seborrhoeic areas, or on sites where it would be difficult to apply a glass slide – such as the interdigital or intertriginous areas – impression samples may be obtained with an adhesive tape. This technique is particularly useful to identify bacterial or Malassezia species overgrowth.
Skin scraping is used to collect material from superficial, plane and dry lesions, such as in cases of dermatophytes skin lesions, or from intensively ulcerative lesions, as in cases of squamous cell carcinoma where the scraping would allow to remove fibrinoid and necrotic tissue. The sixth sampling technique is through a swab, used to sample ear canals, fistulas or intertriginous areas and then rolled on a slide.
After sample collection, slides are commonly stained with Romanowsky-type stains. The one most frequently used in veterinary dermatology is Diff-Quik.
The slide is dipped in an alcoholic fixative and then immersed in two different solutions (eosinophilic and basophilic) for a few seconds.
Samples are then dried at air and observed using a good microscope, which should include four different lens (4×, 10×, 40× or 60×, and 100×). The lower magnifications allows to evaluate cellularity, which must be adequate to formulate a final diagnosis.
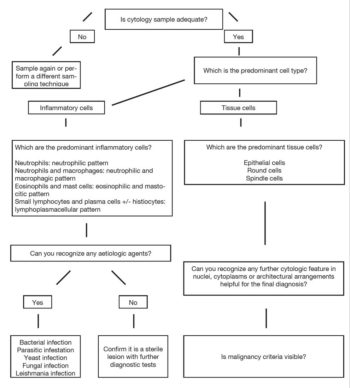
The 40× magnification allows for observation of cells and differentiation between inflammatory or neoplastic lesions. The higher magnification is used to detect small, often intracellular agents, or to evaluate cytoplasmic or nuclear features. The flowchart on cytology sample approach is shown in Figure 1.
If cellularity is adequate, the first step is to discriminate between inflammatory reactions and neoplastic lesions. In inflammatory reactions, the majority of cells will be inflammatory and, depending on which cell predominates, four main inflammatory patterns are recognised: neutrophilic, neutrophilic and macrophagic, eosinophilic and mastocytic, and lymphoplasmacellular.
Whenever a non-sterile process is suspected, the slide must be carefully observed to detect aetiologic agents. In case of neoplastic lesions, cytology mainly yields tissue cells and, depending on their architectural arrangement, tumours can be differentiated into three main groups: round, spindle or epithelial cell tumours.
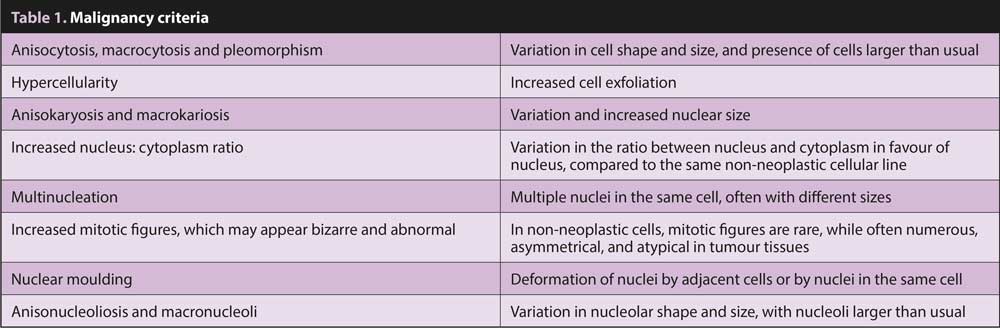
Cell population must be carefully observed to detect possible malignancy criteria to determine if the tumour is benign or malignant. Main malignancy criteria are shown in Table 1.
It is important to note that some tumours may be intensively inflamed, making the differentiation between inflammatory or tumour lesions tricky in some cases.
When the majority of cells identified are neutrophils, the pattern is said to be neutrophilic, and is indicative of acute inflammation.
This pattern is the most common in case of bacterial infection, and neutrophils may show degenerative changes induced by bacterial toxin, such as nuclear swelling, lose of nuclear segmentation (karyolysis) and nuclear streaks. This is typically observed in sampled pustules or collarettes in superficial pyoderma.
A neutrophilic pattern is also observed in cases of non-septic inflammation; for example, in pemphigus foliaceous, the most frequent autoimmune disease of dogs and cats. These pustules contain neutrophils, often showing ageing changes, such as nuclear hypersegmentation and pyknosis (condensation of nuclear chromatin into round aggregates), and many acantholytic cells, which are round, basophilic keratinocytes that have lost their intercellular connection (Figure 2).
In chronic inflammation, neutrophils come with macrophages which are phagocytic cells. Their main role is to “clean” inflammation sites from cellular debris and eliminate aetiologic agents excessively larger than to be eliminated by sole neutrophils.
A neutrophilic and macrophagic inflammatory pattern is recognised in cases of deep pyoderma, furunculosis, fungal infection, parasites (Demodex species), mycobacteria and foreign body reactions. Sometimes, this pattern is called pyogranulomatous when the sample also contains multinucleated giant cells and epithelioid macrophages, which are large round cells that aggregate like epithelial cells.
In the eosinophilic pattern, eosinophils predominate, often associated with mast cells. This pattern is common in some parasitic infestation or in hypersensitive reactions (Figure 3).
Eosinophils and mast cells are rather common in feline skin cytology, and are associated with several clinical manifestations, such as mosquito bite hypersensitivity, miliary dermatitis and eosinophilic granuloma complex diseases. In dogs, this pattern is rather infrequent and mostly observed with sarcoptic mange, flea infestation and facial eosinophilic furunculosis. When small lymphocytes and plasma cells are the main cells present in the sample, the pattern is said to be lymphoplasmacellular. This pattern is uncommon and it is observed in cases of feline lymphoplasmacellular pododermatitis, or in cases of infective agents that strongly stimulate antibody reaction, such as Leishmania infantum.
Plasma cells produce immunoglobulins and, when particularly active, their cytoplasm appears full of large clear vacuoles containing immunoglobulins (Russell bodies); the cell is called the Mott cell. In Leishmania infections, many histiocytes are also present.
The most frequent aetiologic agents identified through cytology, in the skin, are bacteria.
All kinds of bacteria, independently of Gram-positive or Gram-negative, stain in blue, with the exception of mycobacteria.
Cocci appear as small blue dots and, in cases of pyoderma, must be recognised inside the cytoplasm of neutrophils (Figure 4).
When cocci are collected from superficial lesions, are extracellular and in a low number, they may represent normal microflora. Rods are always pathogenic.
Mycobacteria do not uptake Romanowsky-type stains because of their lipid cell wall, but need special stains, such as Ziehl-Neelsen.
Filamentous bacteria, such as Nocardia species and Actinomyces species, are part of the normal oral flora of cats, and are usually recognised in bite lesions.
Malassezia species is a peanut-shaped, sometimes globose yeast, which usually stains in blue and is commonly found in a low number in intertriginous areas, or in the ear canal. Malassezia species overgrowth is a sequelae of primary diseases such as atopic dermatitis, or in cases of feline paraneoplastic syndrome. It causes pruritus, seborrhoea and erythema.
Leishmania infantum can be recognised in infected animals from lymph nodes or through skin cytology from any dermatologic lesions, such as scales, ulcers, pustules, papules or nodules, depending on the different clinical manifestation. It appears as a small oval organism, usually located inside histiocytes or free on the background.
With high-power magnification, it is possible to identify the small rod-shaped kinetoplast perpendicular to the central body (Figure 5).
Dermatophytes may be observed both as septate hyphae, which appear as filamentous bodies adherent to the surface of epithelial cells, and round spores surrounded by a clear halo in the cytoplasm of macrophages, or in the background.
Dermatophytes are usually sampled from plane lesions through adhesive tape apposition or skin scraping, or from nodular lesions (in kerion or in dermatophytic pseudomycetoma), through fine-needle aspiration. Dermatophytes are associated with an intense neutrophilic and macrophagic inflammation.
Cytology also allows for recognition of systemic fungal infection. The most common in Europe is Cryptococcus neoformans, which appears as a round body with a thick capsule, does not absorb Romanowsky stains and appears as a peri-cellular clear halo.
Round cell tumours are characterised by high cellularity. Cells are discrete, round and without intercellular connection.
This group includes mast cell tumours, lymphomas, plasma cell tumours, transmissible venereal tumours (TVT), cutaneous histiocytoma and melanocytoma, the benign counterpart of melanoma. In melanoma, cells may arrange both as epithelial, mesenchymal or discrete cells.
Histiocytic sarcoma originates from histiocytes, but very often shows characteristics of mesenchymal tumours: fusiform cells with cytoplasmic tails and oval-shaped or kidney-shaped nuclei.
In well-differentiated mast cell tumours, cells are characterised by a huge amount of cytoplasmic violet granules, which occasionally obscure the nucleus (Figure 6). These cells are very often associated with eosinophils, which are very helpful to diagnose scarcely differentiate mast cell tumours when granules are low in number, and cells may show several malignancy criteria.
In some cases, fibroblasts may also be numerous.
Benign cutaneous histiocytoma is characterised by discrete cells with a wide pale cytoplasm, which often appears lighter than the background (Figure 7). Cells of TVT resemble histiocytes, but they contain several distinct clear vacuoles, and mitosis is usually numerous.
In cutaneous plasmacytoma, cells are well differentiated plasma cells characterised by a moderate amount of deeply basophilic cytoplasm, and a perinuclear clear area that represents the Golgi apparatus.
In the pleomorphic form, the diagnosis can be difficult due to the huge presence of multinucleated elements and pleomorphic cells with bizarre nuclei.
Lymphoma is composed of a homogeneous monomorphic population of small or medium-sized lymphocytes with scant basophilic cytoplasm. In T cell lymphoma, cells may have cerebriform nuclei.
Neoplastic cells are more fragile and often abound bare nuclei and round basophilic lymphoglandular bodies in the background, derived from damaged cytoplasms.
Melanocytoma is composed of well-differentiated melanocytes containing cytoplasmic, black, fine granular pigmentation. In melanoma, cells show several malignant characteristics, such as anisocytosis, anisocariosis, macronucleoli and pleomorphism. In the amelanotic form, cells lack pigmentation, and the presence of melanophages may help in the diagnosis.
In spindle cell tumours, cellularity is variable. Cells arrange as single elements or are grouped in aggregates by extracellular eosinophilic matrix. Cells are fusiform or spindle, with cytoplasmic tails directed in one or more directions, and contain oval nuclei. Some exceptions exist, such as lipoma: adipocytes are wide round cells containing a single huge vacuole of fat, which does not stain with Romanowsky stains.
Unfortunately, with cytology, it can be difficult or even impossible to differentiate between the several mesenchymal tumours. However, the presence of some specific features can be very helpful; for example, in myopericytoma (formerly called haemangiopericytoma), cellularity is extremely high, cells are characterised by indistinct cytoplasmic border, and often, visible binucleated insect-head cells, multinucleated crown cells and whorls are present (Figures 8 and 9).
Epithelial tumours are characterised by high cellularity; cells distribute in clusters, and usually have distinct cell borders and round to ovoid nuclei (Figure 10).
Cell morphology and architectural arrangement changes depending on the tissue of origin, and may show acinar, ductal or papillary architecture in cases of apocrine gland origin, or cells may have a foamy cytoplasm in cases of sebaceous origin.
In squamous cell carcinoma, keratinocytes may show a fully keratinised cytoplasm while nuclei are rather large, instead of being pyknotic. This phenomenon is called “asynchronous maturation”. In anaplastic carcinoma, cells may appear as single discrete elements.
Summarising, cytology is a very helpful tool in veterinary dermatology and should be performed any time veterinarians deal with cutaneous lesions. It often allows a quick diagnosis of pyoderma, fungal infections or other infectious diseases, such as leishmaniasis.
Moreover, it can orient towards allergic dermatitis in case of eosinophilic inflammatory pattern, or to pemphigus foliaceus when acantholytic cells are sampled from pustular dermatosis. Many skin tumours can be diagnosed with cytology and, even when not diagnostic, cytology orients clinicians towards subsequent diagnostic procedures.