10 Jun 2019

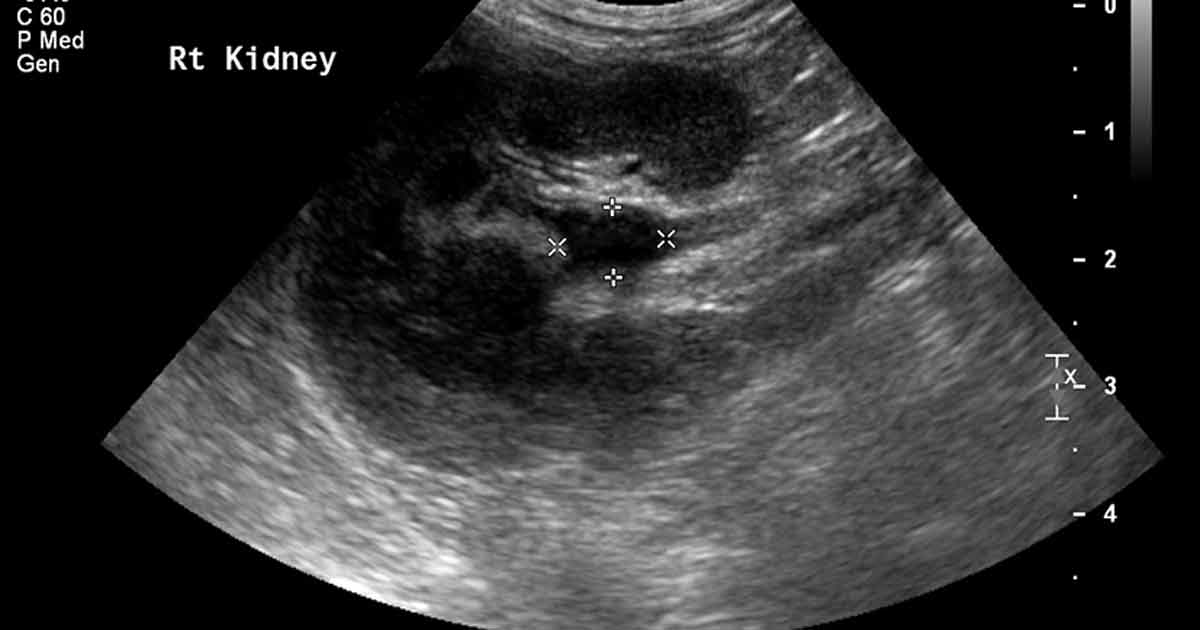
Ultrasound image of the right kidney, showing pyelectasia (renal pelvis dilation) of 8.6mm and surrounding hyperechoic fat.
Kira is a five-year-old, female neutered Tibetan terrier who presented after an episode of vomiting two weeks prior – followed by lethargy, reduced appetite, polyuria and polydipsia (PU/PD).
On examination, she was pyrexic (39.7°C), with caudal abdominal pain.
The rest of the clinical examination was unremarkable (heart rate was 90bpm and respiratory rate 20bpm).
Routine haematology and serum biochemistry showed a mild, mature neutrophilia, mild monocytosis and marginal hyperglobulinaemia. Urinalysis (via cystocentesis) showed adequate concentration; urine specific gravity 1.023 and mild glycosuria. Sediment examination showed pyuria (25 white blood cells/high-power field) and occasional bacteria. Bacterial culture reveals an Escherichia coli infection.
Abdominal ultrasound revealed both kidneys were a normal size and shape, with normal corticomedullary distinction.
The right renal pelvis was dilated (9mm) and the surrounding fat was hyperechoic. The wall of the right ureter was thickened. The urinary bladder contained a moderate amount of hyperechoic debris, with no distal acoustic shadowing. Abdominal radiographs showed no evidence of radiopaque uroliths.
Kira was diagnosed with pyelonephritis. Although the gold standard method to diagnose pyelonephritis involves pyelocentesis and/or renal histopathology, these are rarely used as they are invasive, and a confident presumptive diagnosis can be made using a combination of appropriate clinical signs, laboratory tests and imaging findings.
What is the recommended management and monitoring for pyelonephritis?
Investigations should include evaluation for a predisposing cause of pyelonephritis – such as an obstructive uropathy, chronic kidney disease, urinary anatomical abnormalities, urinary incontinence, neoplasia, immunodeficiency/immunosuppressive treatment, or conditions that alter the composition of the urine (including diabetes mellitus, hyperadrenocorticism and diuretic therapy).
Kira had no evidence of a predisposing factor; therefore, an ascending lower urinary tract infection (UTI) was considered the most likely cause.
The International Society for Companion Animal Infectious Diseases has published detailed guidelines for the treatment of UTIs in dogs and cats. Antimicrobial sensitivity testing should guide appropriate antibacterial therapy, giving consideration to which antibiotics reach high levels in the urinary tract. Treatment should be initiated immediately while awaiting these results. Amoxicillin clavulanate (12.5mg/kg to 25mg/kg) every eight hours is a reasonable choice. Antibiotic therapy may need to be changed based on culture and sensitivity results.
Repeat cystocentesis for bacterial culture should be performed one week into therapy to assess the efficacy of treatment. If a resistant infection is found then minimum inhibitory concentrations may help guide appropriate antibiosis. No strong evidence base exists for the correct duration of antibiosis; however, a minimum of four weeks is recommended.
Cystocentesis for full urinalysis and bacterial culture should be performed one week after cessation of antibiotics to assess whether the infection has been eliminated.
Kira’s PU/PD resolved, and her appetite and demeanour normalised. Repeat urinalysis (via cystocentesis) showed resolution of the bacterial infection in the lower urinary tract, but persistence of the pyuria. The persistent pyuria most likely represented residual inflammation, as repeat urinalysis a few weeks later (after no additional treatment) showed resolution of the pyuria and continued negative culture.