7 Jan 2020

The idea of consuming beneficial microorganisms for health benefits is centuries old. The term “probiotic” was first used in this context in 1974 (Suez et al, 2019).
A panel of the International Scientific Association for Probiotics and Prebiotics slightly modified the original Food and Agriculture Organization of the United Nations/World Health Organization definition of probiotics to “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” (Hill et al, 2014).
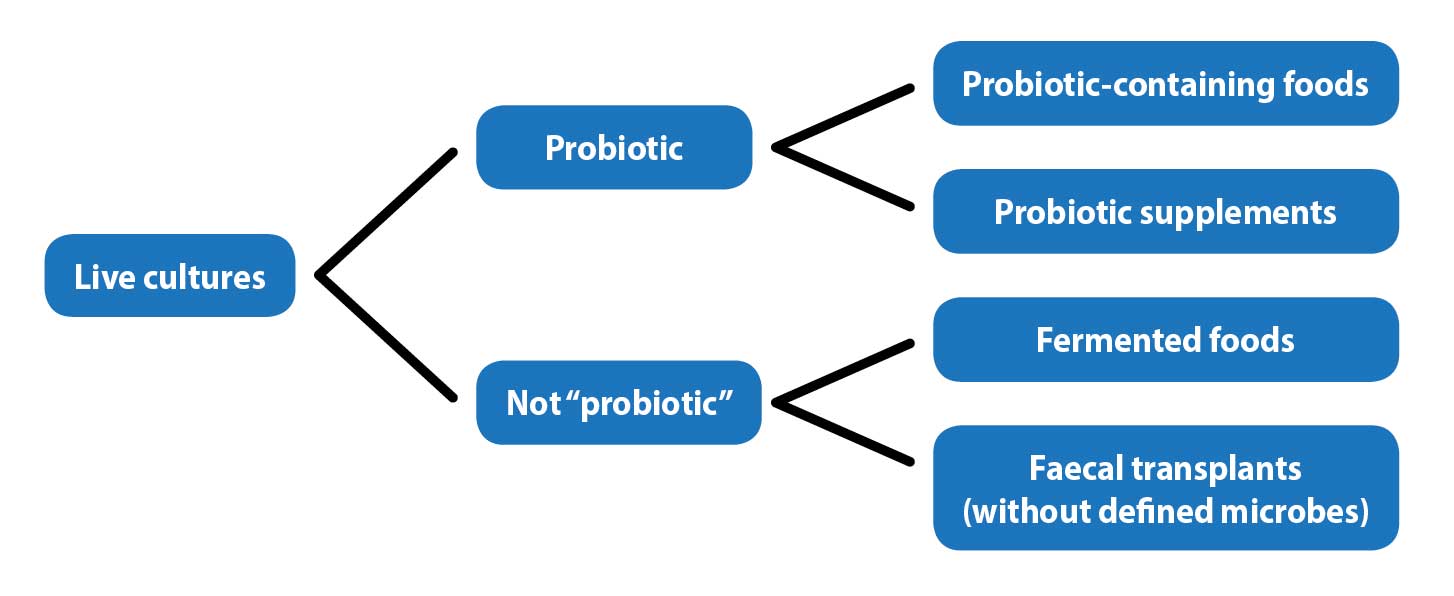
While noting that fermented foods – for example, dairy products – may have benefits, the benefits of the food itself cannot be separated from those of the live microorganisms, so they are not considered probiotics.
Similarly, while faecal microbiota transplantation has shown benefits in humans and veterinary patients, the lack of identification of the microorganisms used excluded this procedure from the definition of probiotic, other than when a defined mixture of microbes is used (Figure 1).
The term probiotic has also been misused for products as diverse as mattresses, shampoos, face creams, aftershaves and even a shampoo that contains yoghurt.
Multiple claims exist for probiotics in humans – including prevention and/or treatment of acute and chronic gastrointestinal disorders, respiratory infections, depression, atopic dermatitis, allergies, diabetes mellitus, obesity and chronic kidney disease (CKD).
Some studies show conflicting results, while some have vague qualitative outcomes – such as self-reported parameters of “well-being”.
Studies are complicated by differences in bacteria and strains, the amount, the host species, and variations within a host species. Individuals vary in their diet, genetics, environment and gut microbiome.
In humans – and likely in animals – the degree of gut colonisation by probiotics is variable as some individuals are resistant to colonisation. One study showed an expansion of the probiotic bacteria in 60% of humans treated and nearly complete resistance in 40% (Zmora et al, 2018).
Most claims for probiotic colonisation use faecal samples. This is an appropriately non-invasive method of collecting samples; however, it may not always represent colonisation in the intestines and is a likely poor indicator of adherence to intestinal cells (Zmora et al, 2018; Suez et al, 2019).
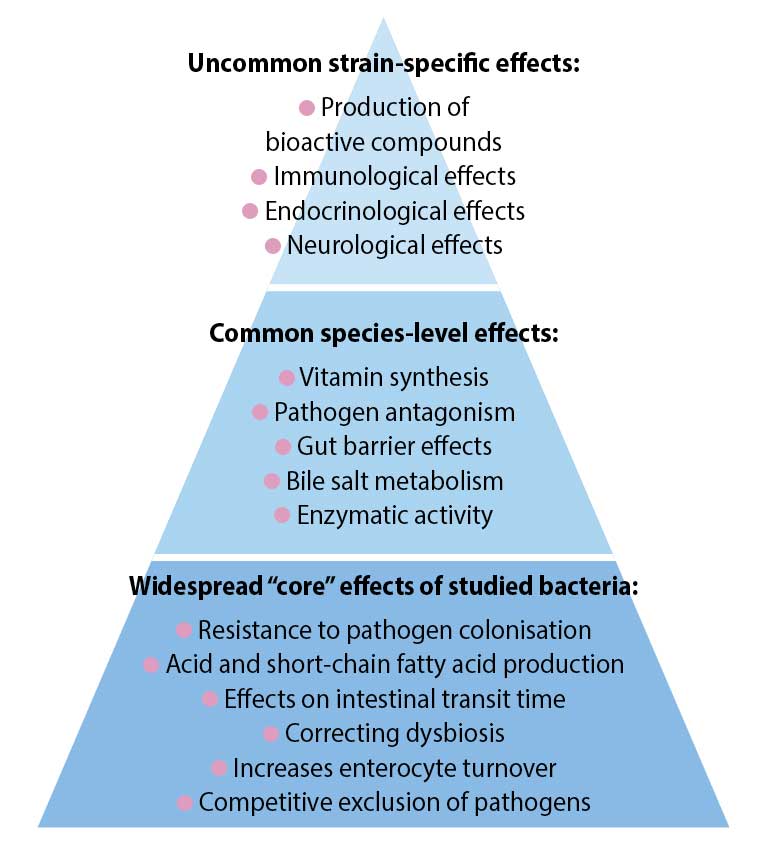
Evidence exists of effects that can be ascribed generally to some probiotic strains if used at a functional dose (Hill et al, 2014; Figure 2).
These include Bifidobacterium species (B adolescentis, B animalis, B bifidum, B breve and B longum) and Lactobacillus species (L acidophilus, L casei, L fermentum, L gasseri, L johnsonii, L paracasei, L plantarum, L rhamnosus and L salivarius).
In humans, the accepted beneficial dose is 1 × 109 colony-forming units per day (Health Canada, 2009; Hill et al, 2014). The intended benefit is supporting a healthy gut microbiota, although this benefit is not well defined.
Human gastrointestinal (GI) disorders where core effects may help include infectious diarrhoea, antibiotic-associated diarrhoea, inflammatory bowel syndrome and ulcerative colitis.
Some of the probiotic mechanisms that may be widespread include resistance of pathogen colonisation, competitive exclusion of pathogens, production of short-chain fatty acids (SCFAs), regulation of intestinal transit, normalisation of perturbed microbiota and increased turnover of enterocytes. Core benefits from non-strain-specific microorganisms have not been described in veterinary patients.
The microbiome is composed of the microbes in – and on – mucosal or skin surfaces and their environment. It is a modifier of disease, an essential component of immunity, influences metabolism and modulates drug interactions (Grice and Segre, 2012).
The GI tract has tens of trillions of microbes, outnumbering host cells tenfold (Schmitz and Suchodolski, 2016). Each individual has its own unique intestinal microbiome – with variation, and an increased density and diversity along the GI tract.
Organisms from the phyla Actinobacteria, Bacteroidetes, Firmicutes, Fusobacteria and Proteobacteria comprise most of the organisms (Jugan et al, 2017).
Microbes ferment fibre to produce SCFAs, including butyrate. Butyrate provides energy for colonocytes, affects the GI tract barrier function, and has anti-inflammatory, antioxidative potential, and potentially anticarcinogenic factors (Patel and DuPont, 2015; Canani et al, 2011).
The intestinal microbiome can be affected by diet, antibiotics and probiotics. Large changes in macronutrients – for example, protein, fat, carbohydrates, fibre or prebiotics – can change the composition of the microbiota (Suchodolski, 2016).
Prebiotics – for example, fermentable fibres – can promote the growth or activity of beneficial bacteria and cause functional changes, such as increases in butyrate (Suchodolski, 2016). Some pet foods include prebiotics – for example, inulin from chicory root or fructooligosaccharides.
Observed changes in the microbiota from probiotics depend on methodology and where the sample is obtained – for example, within the gut or faeces. Probiotics can induce microbiota changes in the large intestine, but these changes are often minor, transient, dose-dependent and host-dependent.
Probiotics administration may result in an insignificant impact on the entire intestinal microbiome. Lactobacillus species increased from 1% to 2.5% of the total bacteria after administration of a multi-species probiotic containing Lactobacillus species (Garcia-Mazcorro et al, 2011).
A mucosa-adherent probiotic may affect the microbiota more significantly; administration of VSL#3 to mice resulted in major changes in ileal microbiota (Mar et al, 2014).
Probiotics may have different beneficial effects, such as immunomodulation, anti-inflammatory properties, or competition for nutrients or adhesion sites with potential pathogens, although the exact mechanisms of probiotics are not fully understood (Ziese et al, 2018).
Acute diarrhoea has many potential aetiologies, including diet changes, infectious diseases or stress. It is often treated empirically without a specific diagnosis (Figure 3).

In dogs with acute gastroenteritis or idiopathic diarrhoea, probiotic treatment with multi-species probiotic, B animalis strain AHC7 or Enterococcus faecium SF68 in conjunction with metronidazole shortened the duration of clinical signs (Herstad et al, 2010; Kelley et al, 2009; Fenimore et al, 2017).
Supplementation of L acidophilus plus the prebiotic inulin to dogs decreased faecal Clostridia species (Strompfová et al, 2013), while Cats given L acidophilus DSM 13241 showed increased elimination of Campylobacter organisms (Baillon and Butterwick, 2003).
E faecium SF68 may have a benefit for kittens with naturally occurring acute diarrhoea, rescue animals with acute diarrhoea, puppies with parvovirus enteritis, and sled and kennelled dogs with stress-related diarrhoea (Czarnecki-Maulden et al, 2007; Bybee et al, 2011; Arslan et al, 2012; Gore and Reynolds, 2012; Kelley et al, 2012; Rose et al, 2017).
Canine acute haemorrhagic diarrhoea syndrome (ACHS) is likely associated with enterotoxigenic Clostridium perfringens and its toxins. In dogs with aseptic ACHS, multi-strain probiotic treatment was associated with a faster normalisation of the intestinal microbiome and a rapid decrease of C perfringens netF toxin genes (Ziese et al, 2018).
Dogs with diarrhoea due to distemper virus were given Lactobacillus murinus, and had improved stool consistency, appetite and mental status compared to placebo, although vomiting was not improved by treatment (Delucchi et al, 2017).
Decreased appetite, food aversion, vomiting and diarrhoea are common in pets given antibiotics. Antibiotics also disrupt the GI microbiome, causing dysbiosis.
In cats given clindamycin, a multi-strain synbiotic improved appetite and the likelihood of completing the treatment course due to less vomiting (Whittemore et al, 2016).
The yeast Saccharomyces boulardii shortened the duration of diarrhoea in dogs given lincomycin and prevented diarrhoea when given concurrently (Atkas et al, 2007).
Cats given amoxicillin clavulanate, along with E faecium SF68, had significantly better diarrhoea scores than control cats (Torres-Henderson et al, 2017).
Dogs with chronic enteropathy (CE) or inflammatory bowel disease (IBD) may have less intestinal microbial diversity than healthy dogs.
E faecium supplementation significantly increases the faecal bacterial richness and diversity of dogs with IBD, and the faecal microbiome become more similar to that of healthy dogs (Schmitz et al, 2014).
Supplementation with Lactobacillus to 21 dogs with food-responsive diarrhoea had beneficial effects on intestinal cytokines and microbiota, but no effect on clinical response as the dogs had already responded to a hydrolysed diet (Sauter et al, 2006).
In 20 dogs with CE, S boulardii improved the clinical activity index, stool frequency, stool consistency and body condition score compared to placebo (D’Angelo et al, 2018).
A multi-strain probiotic given to 10 dogs with chronic IBD decreased clinical and histological scores, and CD3+ T cell infiltration; and normalised dysbiosis (Rossi et al, 2014).
Fewer studies have been reported on probiotics in cats with chronic diarrhoea. A multi-strain synbiotic improved stool quality in adult cats with chronic diarrhoea (Hart et al, 2012). Administration of Lactobacillus group II and E faecium to 27 juvenile cheetahs increased bodyweight and improved faecal quality, compared to controls (Koeppal et al, 2006).
On the other end of the GI disease spectrum, cats with chronic constipation and idiopathic megacolon treatment with a probiotic showed clinical improvement and restoration of the numbers of interstitial cells of Cajal (Rossi et al, 2018).
While some studies for short-term remission of CE have shown some benefits, no reported studies exist on the maintenance of long-term clinical remission of CE.
The dental biofilm – the microbes attached to the tooth surface – is a main cause of dental pathology.
A multi-strain probiotic with L acidophilus LA-5 and B bifidum BB-12 had in vitro bactericidal effects on pathogenic species from supragingival sites of dogs with dental disease (Zambori et al, 2016).
In humans, topical application of Lactobacillus brevis decreased inflammatory mediators involved in periodontitis. Topical L brevis CD2 in dogs reduced gingival inflammatory infiltrates (Vullo, 2014).
This suggests probiotics may be useful in the prevention and control of dental plaque – the cause of periodontitis – although further studies are needed.
The gut microbiota differs between obese and lean individuals. The abundance and diversity of some bacteria may favour different energy and metabolic pathways.
One meta-analysis of human studies on effectiveness of probiotics for weight loss indicated a limited efficacy (Park and Bae, 2015), while another meta-analysis concluded probiotics or prebiotics (but, oddly, not synbiotics) were associated with decreases in body mass index, weight and fat mass (John et al, 2018).
Short-term use of E faecium SF68 in eight cats had no significant effect on food intake, bodyweight, body composition or metabolic parameters in overweight and obese cats (Kathrani et al, 2016; Figure 4).
Further studies are certainly warranted.

In CKD, increased concentrations of uraemic toxins may also cause intestinal dysbiosis, which, with increased intestinal permeability, could potentiate endotoxaemia and low-grade systemic inflammation. These may affect the progression of CKD (Suchodolski, 2018).
In humans with CKD, a six-month probiotic treatment decreased azotaemia (Ranganathan et al, 2009). Similarly, in azotaemic cats, serum urea nitrogen and creatinine concentrations decreased after 60 days of a probiotic – although concurrent treatments varied, and the relationship to quality of life and survival time was not clear (Palmquist, 2006).
In 60 dogs with CKD fed a renal diet, a two-month treatment with the probiotic VSL#3 improved glomerular filtration rate and proteinuria, but had no significant effect on serum creatinine (Lippi et al, 2017).
In 10 azotaemic cats, a multi-strain symbiotic had no effect on azotaemia compared to controls (Rishnew and Wynn, 2011). The same synbiotic did decrease the serum creatinine, but not urea, of 15 azotaemic large cats (6 tigers, 5 lions, 3 cougars, and1 leopard) after six months (McCain et al, 2011).
Oxalate is eliminated through urinary excretion, forming insoluble calcium oxalate in the GI tract with faecal elimination or by oxalate degradation by GI microorganisms.
In humans and rats, probiotics containing Lactobacillus species or Oxalobacter formigenes degrade oxalate in the intestine, resulting in decreased absorption and decreased urinary excretion (Lieske et al, 2005; Zhao et al, 2018).
Dogs with O formigenes in their faeces may have a lower risk of calcium oxalate urolith formation. The prevalence of O formigenes in faeces from dogs with calcium oxalate uroliths was 25%, compared to 50% in healthy dogs and 75% in healthy dogs of breeds not at risk for oxalate uroliths (Gnanandarajah et al, 2012).
Manipulation of GI bacterial components may potentially decrease intestinal oxalate absorption and renal excretion (Weese et al, 2004).
The faeces of 86% of healthy cats had the genes for O formigenes, although the association with oxalate urolithiasis has not yet been explored (Weese et al, 2009).
Further investigations need to determine whether a direct link exists between the lack of oxalate-degrading bacteria and hyperoxaluria, whether their absence is a risk factor for stone formation, and if supplementation can help decrease the risk.
No probiotic products that contain O formigenes are commercially available in the UK for dogs or cats.
Probiotics have been suggested to have a role in allergy treatment – including atopic dermatitis (AD), food allergies, and allergic rhinitis – as the mucosal immune system plays a critical role in the onset of allergic diseases.
Humans and dogs with AD have a skin microbiota dysbiosis, with lower diversity of microbial populations than healthy individuals (Rodrigues-Hoffmann, 2017; (Rodrigues-Hoffmann et al, 2014).
Some evidence exists that AD can be prevented in humans with probiotics, especially if administered during early infancy (Kim and Ji, 2012).
Studies on either the prevention or treatment of canine AD have had mixed results (Marsella, 2009; Kim et al, 2015). One study showed a decrease in the use of prednisolone in dogs with mild to moderate AD given L paracasei K71, compared to those on cetirizine hydrochloride (Ohshima-Terada et al, 2015).
A link exists between the GI digestion capability, the establishment of a specific microbiota and the development of food allergy. Mice orally treated with ovalbumin concomitantly with antacid drugs (which impair gastric peptic digestion) subsequently developed allergic symptoms.
The production of SCFAs from bacterial fermentation can inhibit histone deacetylase-C9 and has prevented asthma development in mice; they may even have an asthma prevention effect in utero (Jensen-Jarolim et al, 2017).
No studies on the use of probiotics for prevention or treatment of asthma, or food allergies, have been reported in cats or dogs.
Probiotics are regulated in the EU as feed additives, even if added as a separate supplement, where they are considered an additive in a complementary food.
All additives must be authorised and are on a list of EU-approved/permitted additives. Feed additives may not be put on the market unless authorisation has been given following a scientific evaluation demonstrating the additive has no harmful effects on human and animal health, and the environment.
Probiotics included on the list are E faecium, Bacillus subtilis, Saccharomyces cerevisiae and several Lactobacillus species.
While generally regarded as safe, it should not be assumed all probiotics are safe in all situations. In humans, possible risks have been described in case reports, clinical trial results and experimental models. They include systemic infections, deleterious metabolic activities, excessive immune stimulation in susceptible individuals, gene transfer and gastrointestinal side effects (Doron and Syndman, 2015).
In humans, case reports exist of fungaemia, sepsis and endocarditis related to probiotic use, including in critical care units (Didari et al, 2014). A risk also exists of probiotic bacteria carrying antibiotic-resistant genes that they could pass to pathogenic bacteria (Imperial and Ibana, 2016).
In humans with severe pancreatitis, treatment with a probiotic appeared to increase the incidence of bowel ischaemia (Besselink et al, 2008), although earlier studies on human pancreatitis did not show this (Olah et al, 2002; 2007).
In mice with induced colitis (but not healthy mice), a butyrate producing bacteria, Anaerostipes hadrus BPB5, accelerated gut bacterial dysbiosis (Zhang et al, 2016).
A review of 622 studies of organisms from Lactobacillus, Bifidobacterium, Saccharomyces, Streptococcus, Enterococcus, and Bacillus concluded no evidence existed of increased risk of disease; however, it was also stated “the current literature is not well equipped to answer questions on the safety of probiotics in intervention studies with confidence…” (Hempel et al, 2011).
No cases of pancreatitis, fungaemia, sepsis or bowel ischaemia due to probiotics have been reported in cats or dogs. One study showed a decrease in serum cobalamin in dogs given E faecium SF68 for two weeks, although another study with Lactobacillus species administered to dogs with food-responsive diarrhoea found no effect on serum cobalamin (Lucena et al, 2018; Sauter et al, 2006).
Where studies have been done, use a probiotic product that has been shown to be safe and useful in dogs or cats for the disorder being treated – for example, E faecium supplementation in cases of acute gastroenteritis may shorten the number of days of diarrhoea, although these cases may also be self-limiting.
Probiotics may have a beneficial role in chronic enteropathies and can be considered as adjunct therapy. Until further research is done, it may be worthwhile to monitor and/or supplement serum cobalamin in these cases.
The other areas of interest have insufficient research at this point to allow for reasonable recommendations – for example, CKD, oxalate urolithiasis, dental disease, AD, allergies, diabetes mellitus and obesity management. These are promising areas for potential benefit.
Research should also include monitoring to ensure safety of long-term use, as this cannot be assumed for all products in all patients.
Probiotics should probably be used with care in pets in a critical care situation or at risk of pancreatitis until more is known.