18 Oct 2021

National media campaigns and increased geographic distribution have raised the profile of Angiostrongylus vasorum, both among pet owners and veterinary professionals. This is important, given the potential severity of disease in infected dogs and its presence in parts of the UK where it had not previously been diagnosed. The effects of other UK lungworms infecting dogs, though, should not be forgotten.
Preventive treatment forms an essential part of canine lungworm control, and geographic and lifestyle factors should be taken into account in establishing whether individual dogs are at risk from infection.
The spread of Angiostrongylus vasorum across the UK in recent years, alongside increasing numbers of products being licensed for its prevention, has led to raised awareness of this parasite among both pet owners and veterinary professionals.
This has been beneficial in reducing canine morbidity and mortality associated with infection – especially in parts of the country where it has not previously been endemic, and infections missed as a result.
The potential severity of angiostrongylosis makes routine preventive treatment for dogs at high risk of exposure essential. Identifying lifestyle and geographic risk factors are key to establishing whether routine treatment is required. When making these assessments, other UK canine lungworms of veterinary significance should also be considered, including Crenosoma vulpis, Eucoleus aerophilus and Oslerus osleri.
A vasorum is a metastrongyloid nematode living in the right side of the heart and pulmonary arteries. It is commonly referred to as “lungworm”, as the most common signs associated with infection are respiratory associated with larval invasion of the lungs.
The life cycle of A vasorum is indirect. First-stage larvae (L1) pass out in the faeces of infected canids and require gastropod molluscs (slugs and snails) as intermediate hosts for further development. Infection occurs in canids when infective third-stage larvae are ingested. This occurs most commonly through deliberate or accidental consumption of infected slugs or snails, although infection can also occur through the consumption of paratenic hosts such as frogs and other amphibians (Morgan et al, 2005).
Infection has also been demonstrated under experimental conditions to occur from ingestion of larvae present in slime trails, but the significance of this in natural transmission is unclear (Conboy et al, 2015; Robbins et al, 2021).
The most common clinical presentation in dogs is mild to moderate pulmonary signs. The most significant of these are coughs (either productive or unproductive) and dyspnoea, with or without tachypnoea. A less common, but more severe consequence of infection is a varying degree of coagulopathy (Morgan et al, 2005).
The mechanism of this aspect of infection is still poorly understood, but can lead to potentially life-threatening signs including anaemia, haematomas, neuropathies, increased and prolonged postoperative bleeding, and post-traumatic haemorrhage. Although less common, these more severe signs can occur even if the parasite is present in low numbers, making routine prevention for the parasite essential in dogs at risk from exposure.
A vasorum has spread rapidly over the past 10 years from endemic foci in Wales, and the south-west and south-east of England across the whole of the UK. Increased reporting of cases has been seen in domestic dogs, with 20% of practices across the country having seen at least one case over a 12-month period (Kirk et al, 2014).
Postmortem surveys were carried out on foxes in 2005 (Morgan et al, 2008) and in 2014 (Taylor et al, 2015), which act as wildlife reservoirs for A vasorum infection. The overall prevalence rose from 7% to 18% in foxes during this period, and extended to regions previously clear of infection such as northern England and Scotland.
This shows the spread of A vasorum across the country is genuine, but not uniform, with focal areas of very high prevalence and other areas remaining free of infection.
Case reporting sites such as the one run by Elanco have been useful in mapping areas of high prevalence and the introduction of A vasorum into new areas. Reporting of cases is only voluntary, however, and distribution of infection very fluid with new foci forming.
It is likely that a variety of factors are driving the spread of A vasorum, including increased movement of dogs around the country – a more favourable climate for increased slug and snail activity.
The potentially fatal outcome if disease is left unchecked makes early diagnosis or prevention favourable. A number of control strategies other than routine preventive treatment exist, but all suffer from limitations. They include keeping dog toys indoors, regular cleaning of outdoor water dishes and not walking dogs in wet conditions when slugs are likely to be active.
It is likely this and similar preventive measures will reduce, but not eliminate the risk of exposure. Picking up and responsibly disposing of dog faeces is an important component of parasite control, and may have local impact – especially as coprophagic slugs may be accidentally ingested by coprophagic dogs. The large fox reservoir of infection means that this is unlikely, however, to be an adequate control measure on its own.
While these measures are likely to have some impact in reducing exposure risk and are worth employing, some exposure in dogs at high risk is still likely to occur. Use of a licensed monthly moxidectin or milbemycin oxime product will minimise the risk of disease in these dogs.
Dogs may be at high risk due to lifestyle. This includes dogs that deliberately eat slugs and snails, and those that may accidentally ingest them through consumption of grass and coprophagia. Some dogs will be at risk due to geographic location.
In areas known to be endemic foci, monthly preventive treatment should be seen as routine – especially prior to surgery. In areas where endemic status is less certain, then testing of dogs prior to surgery, suspected cases and annual testing of young dogs will rapidly build up a picture of whether A vasorum is endemic in an area. This data accumulation is vital if risk-based advice is going to be given.
Diagnostic tests for the detection of A vasorum infection include the following.
Baermann faecal analysis is the gold standard for the diagnosis of A vasorum L1 larvae in faeces. In experienced hands this test is highly specific, but sensitivity of the test relies on the faecal sample collected. Larvae are only shed intermittently in the faeces and if faeces are left on the ground, they will rapidly be contaminated by free-living nematodes from the environment. To maximise sensitivity, faeces must therefore be collected fresh and over three consecutive days.
The test has the advantage of being able to also diagnose O oleri and C vulpis infection as L1 larvae of these parasites are shed in the faeces in a similar way. These can be identified by morphological differences summarised in Table 1, but can be easily misidentified or confused with free-living nematodes. Specificity, therefore, improves with experience.
| Table 1. Morphological characteristics allowing differentiation between first-stage larvae lungworm larval stages in the faeces | ||
|---|---|---|
| Lungworm | Length | Distinguishing features |
| Angiostrongylus vasorum | 334µm to 380µm | Tail has a dorsal notch (Figure 1) and larvae are often coiled (Figure 2). |
|
|
||
|
|
||
| Crenosoma vulpis | 243µm to 281µm | Tail is straight and pointed and larvae straighter in faecal samples, rarely coiled (Figure 3). |
|
|
||
| Oslerus osleri | 229µm to 251µm | Notch in tail after which the distal end has a wavy appearance. |
Faecal smear can be conducted in house with more rapid results, making it useful as an initial screen for parasitic lungworm larvae, but it has a poor sensitivity of 54% to 61% (Humm and Adamantos, 2010).
FLOTAC Is an improved flotation-based faecal counting method for visualising parasite eggs, oocysts and larvae in faecal samples. It has been found to be comparable in sensitivity to the Baermann method for detecting L1 larvae when used with higher specific gravity solutions (Schnyder et al, 2011).
AngioDetect (Idexx Laboratories, US) is a point-of-care blood test that detects circulating A vasorum antigen in the blood. It has a reported sensitivity of 84.6% and specificity of 100% (Schnyder et al, 2014). This test allows for more rapid diagnosis in a clinical setting, and also allows many dogs with clinical signs compatible with A vasorum infection to be tested relatively economically and rapidly.
Bronchoalveolar lavage will often yield larvae in infected dogs, but carries some risk in dogs suffering from respiratory compromise. It is not practical for use as a routine test.
C vulpis has a similar life cycle to A vasorum, with foxes also acting as a wildlife reservoir. Adult worms (Figure 4), however, live in the bronchi. A prevalence of infection of 10.8% has been demonstrated in British foxes (Taylor et al, 2015), but this has translated into only sporadic cases in domestic dogs. This may be in part due to less awareness of this worm among vets when compared to A vasorum.

In most cases, clinically affected dogs present with mild respiratory signs, such as bronchitis with mucopurulent discharge and chronic cough, but these can become severe enough with the potential for secondary respiratory complications to occur (Conboy, 2009).
Adult worms and larvae may be found by bronchoalveolar lavage, or larvae identified in fresh, pooled faecal samples, by Baermann or FLOTAC. Moxidectin/imidacloprid spot-on solutions and milbemycin oxime tablets are both licensed for the treatment of C vulpis infections, and are likely to have at least some efficacy in disease prevention.
Due to the similar life cycle and epidemiology to A vasorum, control methods for C vulpis other than preventive treatment have similar limitations. Risk factors for infection are similar to A vasorum and so if preventive treatments are put in place for A vasorum, this should also be sufficient for C vulpis.
E aerophilus (C aerophila) is primarily a parasite of the fox respiratory tract. Domestic canine and feline respiratory infection caused by E aerophilus is sporadic across Europe, and most cases are subclinical. Clinical cases in dogs and cats, however, have been reported (Foster et al, 2004).
Knowledge of host range and geographic distribution of E aerophilus in the UK and Europe is fragmentary, but dogs and cats are occasionally infected due to reservoirs of infection maintained in fox wildlife hosts where prevalence can be high. The life cycle is direct, with eggs being passed in the sputum or faeces and reaching the infective embryonated stage in 30 to 50 days.
Larvae from ingested eggs penetrate the intestinal mucosa and migrate to the lungs. They then penetrate the alveoli, migrate up the airway, and reach maturity in the bronchi and trachea, where the adult worms live. Typical clinical signs associated with infection include a cough (productive or unproductive), sneezing and dyspnoea.
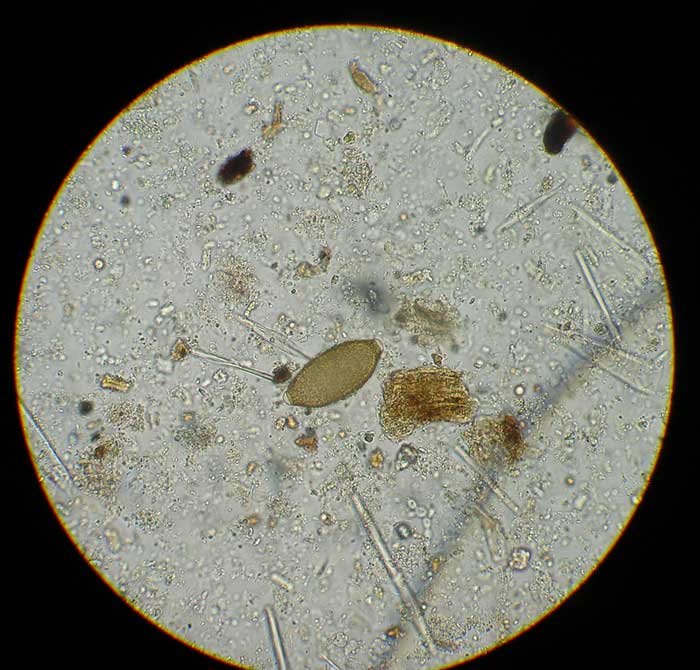
Diagnosis is achieved by identification of the lemon-shaped, slightly asymmetrical bipolar plugged eggs (60µm by 30µm; Figure 5) by faecal flotation. Shedding of these eggs may be intermittent and faeces should be tested over three consecutive days.
Prevention of exposure to infection is impossible in pets with access to the outdoors, but is not essential, other than in those who are repeatedly infected. In these cases, or in focal outbreaks, the use of a monthly moxidectin/imidacloprid spot-on preparation should be considered.
Moxidectin/imidacloprid is licensed for the treatment of E aerophilus in cats and E boehmi in dogs – and, in the author’s opinion, is likely to have some preventive efficacy.
O osleri is a parasite of the upper bronchi of dogs with adult worms living in granular nodules. These are most commonly found at the first bronchial bifurcation. Clinical cases are sporadic in the UK and the overall prevalence is unknown as many cases are sub-clinical.
The life cycle is direct, with the L1 larvae passed in the faeces or sputum. These larvae are infective to other dogs and build up quickly in warm humid environments – especially those with high densities of dogs. Clinical outbreaks, therefore, tend to occur most commonly in kennels and other forms of communal housing.
Clinical signs, if present, are often mild, but a chronic cough and exercise intolerance can develop from prolonged infection. Nodules containing adult worms can be visualised by bronchoscopy and are pathognomonic. Larvae can also be detected in fresh faecal samples pooled over three days, by using Baermann apparatus or FLOTAC.
No licensed preventive treatment exists and clinical outbreaks can be prevented by maintaining dry, clean housing, and prompt picking up and disposal of faeces to prevent environmental contamination with larvae.
Preventive treatment for lungworm infections in dogs need to be considered as part of an overall parasite control programme. Lifestyle and geographic risk factors need to be assessed in establishing whether routine preventive treatment is required.
In the case of A vasorum, testing suspected cases alongside routine testing of young dogs and those requiring surgery is an important part of assessing whether A vasorum is present in a particular area. While obtaining, recording and sharing this data is vital to help map the distribution of A vasorum in domestic dogs, the fluid nature of endemic foci must be remembered and vets not become complacent if they have not yet seen cases in their locale.
More information on the epidemiology of other types of lungworm in the UK is required, making preventive advice difficult, but they should still be considered – especially if practices have seen cases locally, individual dogs have a history of infection or there have been local outbreaks.