21 Feb 2025

Image: © Mauricio/ Adobe Stock
The benefits of routine faecal testing for intestinal nematodes in cats and dogs is being increasingly recognised, alongside routine preventive treatment or as a replacement for it. Confusion, however, does exist surrounding positive and negative results and how they should be interpreted.
Testing for Giardia species and tapeworm in healthy patients can also produce results that are difficult to interpret. By understanding the tests available and the parasites being identified, we can decide on whether treatment is necessary and plan further preventive treatment strategies.
Regular deworming of cats and dogs has long been incorporated into pet health programmes to reduce pet disease and zoonotic risk. In comparison, routine screening for intestinal parasites in the UK has remained largely overlooked.
This has started to change recently, with concerns over drug resistance going undetected in worm populations in UK pets alongside the potential for environmental contamination with anthelmintics.
While many benefits exist for routine screening for intestinal parasites, it is important to know what the aims in testing are, as well as being able to interpret the significance of a positive or negative result.
Ascarid (Toxocara species and Toxascaris species) and hookworm (Ancylostoma species and Uncinaria species) infections are the most common intestinal worm infections found in UK cats and dogs, with whipworms (Trichuris vulpis) also occasionally seen in dogs. Ascarid infections are generally well tolerated, but large burdens can lead to intestinal obstruction, and migrating Toxocara larvae can cause respiratory signs in puppies and kittens. Zoonotic potential from Toxocara species eggs shed into the environment must also be considered.
Hookworm infections can cause or contribute to clinical disease, with diarrhoea, weight loss and anaemia being the most common clinical signs. Skin lesions can appear on the pads of dogs and cats caused by larval penetration. All Ancylostoma species can also cause significant anaemia when present in high numbers or over a period of time. This can be severe and life threatening in pups infected by lactogenic transmission.
Uncinaria stenocephala is the most common hookworm found in UK dogs (Wright and Wolfe, 2007; Wright et al, 2016) and is less pathogenic than Ancylostoma species. It can, however, contribute to or cause diarrhoea and gut malabsorption. T vulpis is uncommon in UK dogs, but can cause outbreaks of disease in kennels and breeding establishments where build up of infective eggs in the environment can lead to heavy worm burdens. These infections can lead to colitic diarrhoea, weight loss, tenesmus and, less commonly, rectal prolapse.
Routine treatment on the basis of risk assessment is employed in cats and dogs to reduce worm burdens and egg shedding with the aim of limiting zoonotic exposure and clinical disease. It is also crucial to reduce build up of egg contamination in enclosed groups such as breeding establishments and kennels when clinical outbreaks occur. Routine screening alongside this treatment is beneficial for early detection of drug resistance, as well as assessing owner compliance and that medications are being administered at the correct dosage and at an adequate frequency.
Routine testing can be performed instead of routine treatment to detect pets that are infected and then treat as required. This reduces anthelmintic use, but also allows for potential shedding to occur in between treatments. It is important that pet owners appreciate this zoonotic risk when agreeing to this strategy.

The more often routine screening is carried out, the less likely is the chance of zoonotic Toxocara species eggs being shed into the environment in between tests or parasitic life stages building up in the environment.
Little data exists to suggest what an optimum testing frequency would be. If being used instead of routine treatment, then the frequency would be initially gauged on the degree of risk. Indoor cats pose very low risk, and once a year testing is sufficient. Pets with outdoor access should be initially tested at least four times a year and, ideally, monthly for high-risk groups. If no shedding is found over time, then this frequency can be decreased and similarly increased if frequent shedders are identified.
When screening alongside routine treatment is used, then testing can be coincided with annual or six-monthly health checks to monitor efficacy of treatment plans over time. Testing can be incorporated into practice health plans to spread cost.
A variety of diagnostic tests for canine and feline intestinal roundworms are available.
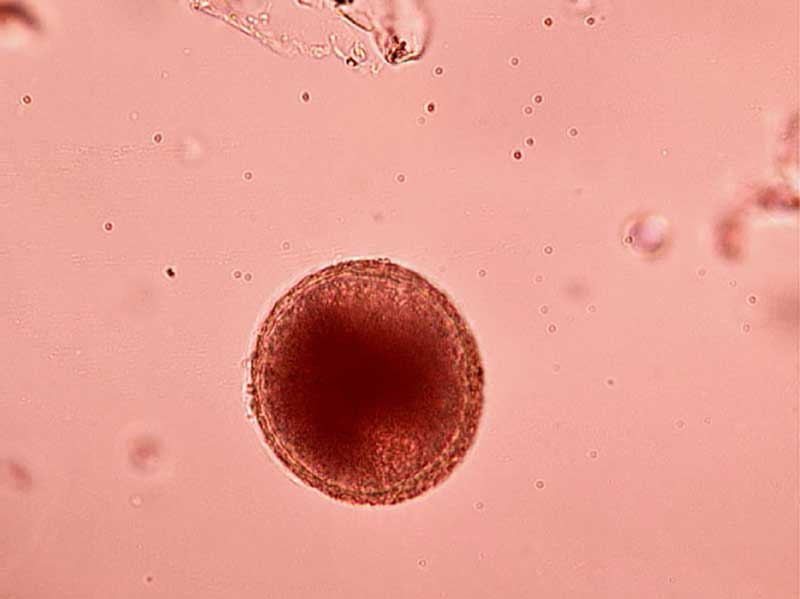
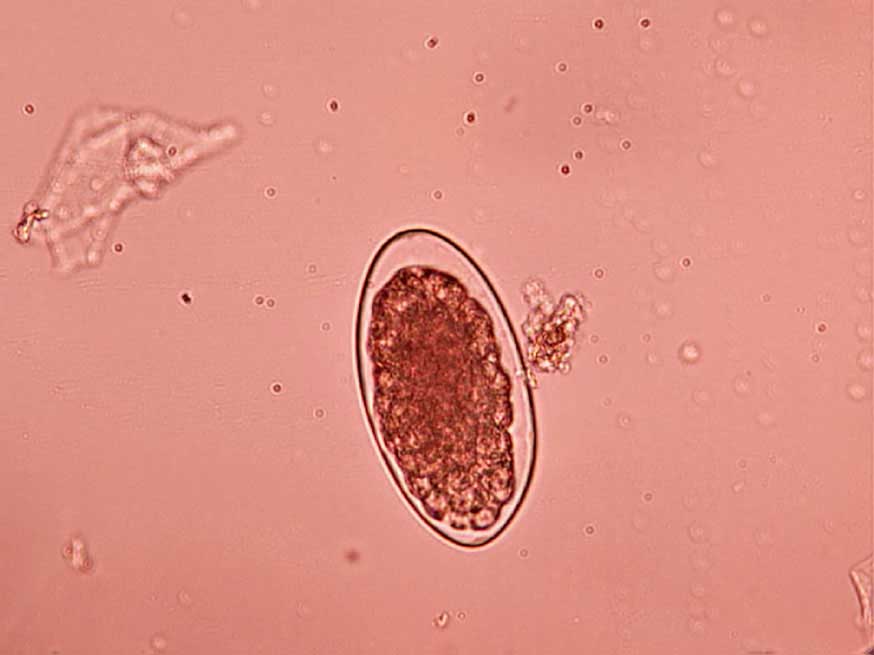
Faecal flotation: Shedding of ova is often intermittent so, ideally, samples should be collected over three consecutive days for increased diagnostic sensitivity. Toxocara species eggs (Figure 1), hookworm eggs (Figure 2) and Trichuris species eggs may all be detected by faecal examination methods. Faecal flotation allows much larger volumes of faeces to be examined by concentrating ova present while eliminating debris. This technique may still have diagnostic sensitivities as low as 60 per cent for some roundworm ova such as Toxocara species and has poor sensitivity for tapeworm egg detection (Wolfe et al, 2001). It is important to know whether dogs are coprophagic prior to testing, as this can lead to false-positive results. This is due to eggs from cat and dog faeces or strongyle eggs in ruminant and horse faeces being present, which will pass through the digestive tract of cats and dogs unchanged. Many different flotation methodologies are described in the literature, and AI can now also be employed to aid with sensitivity and specificity. A summary of the technique can be found in the European Scientific Counsel Companion Animal Parasites’ diagnostic guideline 4.
Coproantigen testing: Faecal antigen testing is commercially available in the UK for intestinal ascarids, hookworms and whipworms. These tests allow infections to be detected when ova shedding is not occurring and avoids false-positive results due to coprophagia. They give no indication, however, to what extent ova shedding may be occurring at any given time.
PCR: Like faecal antigen testing, PCR is commercially available in the UK and has a high sensitivity and specificity for roundworm detection. It also does not assess egg shedding, just the presence of the parasite.
When controlling outbreaks in breeding or kennel establishments, positive dogs should always be treated and then retested to minimise environmental shedding. In domestic situations, the question is whether to allow shedding to reduce the risk of anthelmintic resistance developing.
Resistance is not reduced by not treating negative pets. It is only reduced by deliberately allowing the shedding of parasitic life stages into the environment. If low numbers of hookworm and Trichuris species are present, or low numbers of ova are being shed, faecal hygiene is good, and pets are clinically healthy, then it is an option to allow this to occur.
In the case of Toxocara species infection, however, any shedding of ova represents zoonotic risk, which presents an ethical issue in suggesting shedding should be allowed to occur. Even if individual pet owners prepared to sign off on this risk, potential zoonotic risk to the wider public exists.
On this basis, any positive cat or dog identified should be treated. If positive results are identified for pets on treatment, potential compliance and administration issues should be explored. If positive results are occurring between treatments, then frequency could potentially be increased.
The dosage should also be checked to be correct, and for faecal flotation, the possibility of coprophagia considered. Once all of these options have been considered, then drug resistance becomes a possibility and should be investigated – especially if repeated positive results are being identified after treatment.
The sensitivity of individual faecal flotations can be low, but even in tests with higher sensitivities, trends should be followed in individual patients over time.
Negative results over time indicate adequate parasite control and/or low exposure. If faecal testing is being performed instead of routine treatment, and negative results are repeatedly obtained over time, then frequency of testing could be reduced, considering any lifestyle changes which may also occur.
The most common tapeworms of UK cats and dogs are Taenia species and the flea tapeworm Dipilidium caninum. Dogs may also be infected with Echinococcus granulosus.
Tapeworm infections are generally well tolerated by cats and dogs, but high burdens can lead to loss of body and coat condition, and in extreme cases, intestinal obstruction. Motile segments crawling out of the anus can also lead to anal pruritus. Taenia species and E granulosus infection in dogs can lead to economic losses through meat and offal condemnation due to livestock consuming eggs from faeces contaminating pasture and feed stores.
D caninum is zoonotic through accidental infected flea consumption. Cases are uncommon and can be largely avoided by good hygiene and adequate flea control. E granulosus is a serious zoonosis if eggs passed in faeces are ingested via faecal contamination.
Faecal flotation has a very low sensitivity for tapeworm, and no faecal antigen or PCR is available for Taenia species detection. Routine treatment for high-risk cats and dogs is, therefore, an important part of control with faecal testing being currently a poor alternative. Although faecal PCR is available for Echinococcus species, ova passed in faeces are immediately infective, and so testing should not be used for Echinococcus species as an alternative for routine treatment of high-risk dogs. It is useful as part of routine testing, however, to monitor where the parasite is present in the UK and whether control measures in individual dogs are effective.
Routine flea control should be adequate for D caninum control. Faecal antigen testing for D caninum is available as part of a worm testing package, however, so it is important to be able to correctly interpret a positive or negative result.
Positive cats and dogs for Taenia species tapeworm should be treated to prevent high burdens developing and tapeworm segment shedding eroding the human-animal bond. This will also prevent ruminant pasture and feed store contamination in the case of canine Taenia species.
Positive Echinococcus species dogs should be treated immediately and their coats washed to minimise any coat contamination with eggs. Good hand hygiene should be maintained until the infection is cleared and faeces disposed of responsibly.
Positive faecal antigen tests for D caninum are good indications that flea control has been lost and should be investigated. Positive pets should be treated and flea exposure minimised to try to avoid further infection.
Negative results for D caninum indicate good flea control. The poor sensitivity of available tests for Taenia species means that little can be interpreted from a negative result, and decisions to treat should be based on lifestyle factors. A negative result for Echinococcus species means that current treatment is effective.
For those not on treatment, a negative result does not mean that exposure may not occur, and lifestyle and geographic factors should continue to be assessed when deciding if routine treatment is required.

Giardia duodenalis (also known as G intestinalis) is a flagellate protozoan which inhabits the intestines of a variety of domestic and wild animals. Infection can cause diarrhoea and weight loss – especially in young patients or the immunosuppressed.
Some subtypes of G duodenalis have zoonotic potential, but risk in immunocompetent patients is low. Infection can be subclinical in both cats and dogs, and prevalence of infection high (Bouzid et al, 2015). This can make it difficult to establish the significance of infections found in both subclinical patients and those with gastrointestinal signs.
With possible Giardia species resistance developing to chemotherapeutic agents (Lalle and Hanevik, 2018), careful consideration should be made as to whether to treat subclinical carriers, and routine screening is not indicated unless a specific goal exists, such as trying to remove the parasite from breeding or kennel premises.
The following methods are all useful in the diagnosis of Giardia species infection.
Direct smear examination of fresh faeces: Direct smears can be used for the detection of Giardia species life stages passed in the faeces. A small quantity (about the size of a match head) of fresh, warm faeces is mixed with saline. Active trophozoites may then be identified. Giardia species trophozoites have a “falling leaf” motion with a concave sucker and two nuclei. The need for fresh faeces to be examined quickly and kept warm makes this method of diagnosis impractical in many clinical situations, so faeces are often examined for faecal cysts instead. The sensitivity of examination for Giardia species cysts by direct smear is low, and faecal flotation techniques are required to improve sensitivity.
Faecal flotation: Although this increases sensitivity, faecal samples should be collected on at least three consecutive days, as shedding of cysts is often intermittent.
Antigen detection in faeces: Highly sensitive faecal antigen ELISA patient side tests are now commercially available. These are convenient for use in the practice setting and have a high sensitivity. They will, however, detect recent and current infection. Low parasite burdens detected may also not be clinically relevant.
PCR: PCR tests for Giardia species infection are also now commercially available. These carry high sensitivity and specificity in the same way as faecal antigen detection. Some PCR tests also allow the zoonotic potential of infections to be established.
Flotation techniques tend to underestimate clinical infection and, in contrast, antigen and PCR testing overestimate them. Flotation used in combination with one of these other tests is, therefore, extremely useful to confirm if infection is present or persisting after treatment and the degree of cyst shedding that is taking place.
Diagnostic testing should be repeated in animals after treatment to see if shedding is still taking place, and where clinical signs have not improved to establish if infection has been eliminated. This should be done no more than three days after the completion of treatment to establish if infection is persisting rather than infection having reoccurred from the contaminated environment. This is important, as many multi-pet and individual infections prove difficult to clear because of insufficient environmental hygiene rather than lack of treatment efficacy.
The Companion Animal Parasite Council recommends performing a faecal flotation with centrifugation primarily for detection of cysts after treatment rather than faecal antigen tests alone. This is because faecal antigen tests may remain positive even after treatment for variable periods of time, even if infection has resolved.
Treatment is not indicated in positive subclinical pets unless concern exists about significant immunosuppression in humans contacting the pet or if attempts are to be made to eliminate infection from multi-pet premises.
In clinical cases, treatment should be initiated with the aim of resolving clinical signs rather than infection. It is useful to test post-treatment as described to establish if infection is still present and a possible cause or recurrent diarrhoea. Other underlying causes of gastrointestinal signs should also be investigated, as Giardia species is often not the sole cause – especially in chronic diarrhoea and in older animals.
Faecal antigen has a very high sensitivity, so it can be assumed that infection is not present.
Negative flotation can result in false -negative results, so faecal antigen is recommended to confirm absence of infection where diarrhoea is persisting or where infection is being eliminated from premises.
Screening for intestinal parasites is beneficial in establishing efficacy of treatment, preventive programmes and as an alternative to routine treatment.
It is important to be clear, however, regarding the aims of testing and its limitations for some parasites.
By understanding both the parasite being diagnosed and the test being used, testing can be employed effectively and interpreted correctly.