8 Mar 2022

Image © FurryFritz / Adobe Stock
Hyperthyroidism is the most common endocrine disease of older cats.
Diagnosis is frequently straightforward, with most cats presenting a typical history and examination findings, and a high index of suspicion will be maintained for this well-known disease.
However, various other common conditions exist that can mimic hyperthyroidism, and optimum interpretation of thyroid function test is key to both successfully identifying more subtle cases and avoiding over-diagnosis of the condition.
Early curative treatment (surgical thyroidectomy or radioactive iodine treatment) should be considered for a hyperthyroid cat, as this brings the best prognosis, removes the possibility of transformation from adenoma to carcinoma, and minimises subsequent cardiovascular and renal consequences.
Additionally, long-term owner and patient compliance are no longer a consideration once the condition has been treated. However, the individual circumstances of the cases, including both owner and patient factors, and available facilities and surgical expertise, are always a consideration, and some cases exist where life-long medical management will be the preferred option.
Over the years, an increased prevalence, and/or awareness of feline hyperthyroidism, has led to more subtle or atypical signs relating to the condition being detected much earlier by either owner or clinician.
In these early cases, or those compounded by non-thyroidal illness, a greater diagnostic challenge exists. A wider appreciation of the utility of free thyroxine (FT4), alongside total thyroxine (TT4) aided diagnosis of such cases where the TT4 might often be non-diagnostic when evaluated in isolation. Latterly, though, it is the author’s experience that the diagnostic value of TT4 and FT4 may be overestimated, leading to a substantial rate of “positive” misdiagnoses.
A wide age range is reported for spontaneous hyperthyroidism in cats (4 to 20 years), but most patients are above the age of 10 at presentation. Typical historical findings are shown in Table 1.
| Table 1. Presenting signs in feline hyperthyroidism | |
|---|---|
| Common | Uncommon |
| Weight loss | Decreased appetite |
| Polyphagia | Lethargy |
| Vomiting | Dysponea/panting |
| Polyuria/polydipsia | Asymptomatic |
| Hperactivity | Weakness |
On examination, weight loss is usually confirmed, the coat may be unkempt, and the patient may appear jittery or hyperactive.
Tachycardia is a frequent finding, often accompanied by a murmur or gallop sound. Under the stress of travelling and examination, tachypnoea may be more pronounced (Figure 1).
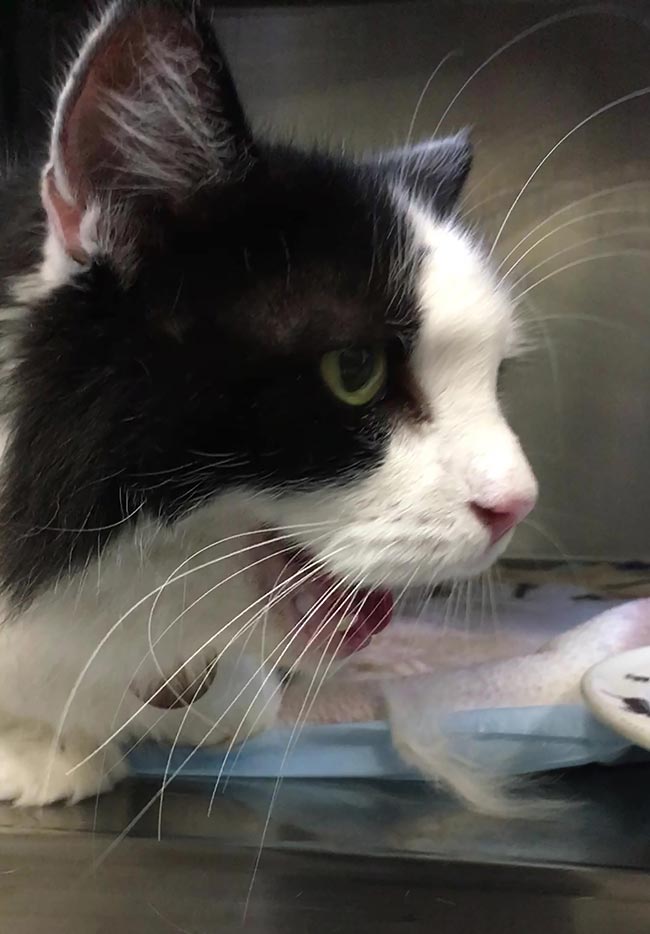
A goitre is usually palpable on one or both sides of the trachea. However, non-functional goitres are also well-described and, conversely, as goitre detection is a learned skill and functional thyroid tissue is sometimes located within the thorax, the apparent absence of a thyroid mass should not necessarily mean a diagnosis of hyperthyroidism is discarded.
Various in-clinic machines have the capability to measure TT4, but it should be noted that agreement with the reference laboratory assessment is not always perfect, and, in particular, a tendency exists for some methodologies to occasionally overestimate TT4.
TT4 is otherwise very specific and it is moderately sensitive.
In early or mild hyperthyroidism, normal fluctuation of TT4 may bring the hormone level into the normal range at the time of measurement, so potential exists for a false negative result.
TT4 generally shows a gradual decline with age; a high-normal or even mid-normal TT4 in a geriatric cat could be regarded as suspicious for hyperthyroidism if there are compatible signs.
In cases where concurrent non-thyroidal illness is present or under the influence of certain medications, TT4 may be suppressed into the upper half of the normal range.
In these situations, FT4 may be informative. FT4 is the biologically active form of thyroxine, and this parameter tends to be more consistently raised in hyperthyroid cats, even with non-thyroidal illness. However, it is not a suitable first-line test for hyperthyroidism due to its lower specificity, with occasional sick euthyroid cats showing elevation of FT4 (Peterson et al, 2001). Therefore, FT4 results should always be interpreted in conjunction with a total T4, rather than relied upon in isolation.
Mild FT4 increase may be of little diagnostic value. A significantly elevated FT4 will be supported by a high-normal TT4 in a hyperthyroid animal, but in a patient with a high FT4 and low-normal or sub-normal TT4, a euthyroid sick status should be suspected. In this context, results of TT4 from any test methodology that may provide an overestimate must be viewed with a particularly critical eye.
Crucially, it is essential to not lose sight of the clinical case seen as a whole; how suggestive are the signs and examination findings (Figure 1)? Are there any other possible (potentially more likely) causes of the presentation, and have these been adequately excluded (Table 2; Figure 2)?
| Table 2. Non-exhaustive list of differential diagnoses for hyperthyroidism | |
|---|---|
| Clinical finding | Some alternative differentials |
| Weight loss and/or polyphagia | Diabetes mellitus, chronic kidney disease (CKD), intestinal disease, inflammatory hepatobiliary disease, cancer cachexia |
| Tachycardia | Fear, stress, primary cardiac disease, anaemia |
| Polyuria/polydipsia (PD) | PD only secondary to diarrhoea, hyporexia of canned food with compensatory increased water intake to maintain fluid balance Diabetes mellitus, CKD, hypercalcaemia |
| Behaviour change | Hypertension, musculoskeletal pain, cognitive dysfunction syndrome, other central disease |
| Apparent goitre | Parathyroid gland, non-functional goitre, granuloma, lymph node |

Considerable inter-patient variation in thyroid hormone levels exists (Jordan et al, 2021), and, if in any doubt, it is better to follow a patient’s own thyroid levels over time, rather than risk misclassifying a cat as hyperthyroid or euthyroid based on a single set of borderline results.
Canine thyroid stimulating hormone (cTSH) measurement, in conjunction with other tests, may be of diagnostic value in detecting early or mild cases of feline hyperthyroidism, or those compounded by non-thyroidal illness, but its greater asset is in aiding exclusion of hyperthyroidism in euthyroid cats that show signs suggesting hyperthyroidism.
A measurable cTSH is very unlikely to occur in a hyperthyroid cat (0% and 2% of cases in two published studies; Williams et al, 2014; Peterson et al, 2015), such that this could be used as a rule out for hyperthyroidism in equivocal cases.
As a method of ruling in hyperthyroidism, a cTSH sample is non-specific, but the addition of a TT4 (or FT4) can increase diagnostic accuracy to near 100% (Peterson et al, 2015). Additional points to note about thyroid function tests are summarised in Table 3.
| Table 3. Comparison of thyroid function tests for the diagnosis of hyperthyroidism | ||
|---|---|---|
| Parameter | Advantages | Limitations |
| Total thyroxine | Specific | Limited sensitivity in certain cases. In-clinic measurement may not correlate perfectly with reference laboratory result. |
| Free thyroxine | Sensitive | Poor specificity. Must be run by equilibrium dialysis (only reliable method). Expensive. Sample storage can lead to false increases. |
| Canine thyroid-stimulating hormone (cTSH) | Excludes hyperthyroidism if detectable | Non-specific if used alone as one in three euthyroid cats have undetectable cTSH. Normal reference ranges not fully established in cats. |
| Total triiodothyronine | None | Poorly sensitive. Not widely available. |
Finally, as part of a more in-depth investigation into concurrent disease, haematology and biochemistry may often be undertaken. Results will also serve to aid the diagnostic process in suspect cases of hyperthyroidism; presence or absence of various changes, although non-specific, may increase or decrease suspicion for the condition (Table 4; Figure 3).
| Table 4. Typical clinical path changes in cats with hyperthyroidism (most common changes are in bold) | ||
|---|---|---|
| Haematology | Biochemistry | Urinalysis |
| Erythrocytosis (mild) | Increased liver enzymes | Submaximal concentration |
| Increased Heinz bodies | Increased phosphate | Proteinuria |
| Neutrophilia | Increased urea | Bacteriuria (often asymptomatic) |
| Lymphopenia, eosinopenia | Low-normal creatinine | |
| Lymphocytosis, eosinophilia | Hypokalaemia | |
| Reduced fructosamine | ||
| Increased bilirubin (mild, rare) | ||

Dynamic tests are rarely performed or required in hyperthyroid patients. Frustratingly, they often do not add much in the patients where supplementary tests may be considered (that is, in those with concurrent hyperthyroidism and non-thyroidal illness).
Usually, a better approach is to watch and wait, identify and control any non-thyroidal illness if present, and then retest for hyperthyroidism.
The thyrotropin-releasing hormone (TRH) stimulation test is rarely carried out since the withdrawal of medical grade TRH from the market several years ago. Also, the administration of TRH could cause dramatic (although transient) cholinergic side effects (such as salivation, tachypnoea, micturition, vomiting and diarrhoea).
Evidence exists that shows the triiodothyronine (T3) suppression test may occasionally be useful in mild/borderline hyperthyroid cats (see Table 5 for protocol).
| Table 5. Protocol for Triiodothyronine (T3) suppression test in cats | ||
|---|---|---|
| Day | Action | Measurement |
| 1 | Collect and store serum sample. | T3 and total thyroxione (TT4) |
| 2 | T3 administered: for cats less than 5kg, 20µg by mouth every eight hours. For cats more than 5kg, 30µg by mouth every eight hours. |
N/A |
| 3 | T3 administered: for cats less than 5kg, 20µg by mouth every eight hours. For cats more than 5kg, 30µg by mouth every eight hours. |
N/A |
| 4 | Final T3 dose administered in morning. Collect serum sample two to six hours later. Send with day one sample. |
T3 (ensure compliance) TT4 (diagnosis) |
In normal cats, exogenous T3 suppresses pituitary TSH production and, therefore, TT4 levels. In hyperthyroid cats, TT4 production is autonomous and TSH is already suppressed (negative feedback). Exogenous T3 administration has a negligible effect on T4 levels.
Comorbidities may not only confound diagnosis, but also influence decisions for management. Questions to be answered include:
Baseline renal function and blood pressure should be assessed, as hyperthyroidism can significantly affect the renal and cardiovascular systems due to an increase in circulating catecholamines and metabolic rate, and a resultant increase in cardiac output.
Restoration of a euthyroid state generally improves the cardiovascular status, but careful reassessment is prudent; the possibility exists for concurrent primary hypertrophic cardiomyopathy or hypertension due to some other underlying disease. In one study, a little more than 20% of cats went on to develop hypertension despite good control of hyperthyroidism over a six-month period (Syme and Elliot, 2003). The cause of this was not always clear; chronic kidney disease (CKD) was not always conclusively responsible.
Around 10% to 14% of cats to have evidence of concurrent CKD at the time of diagnosis of their hyperthyroidism. These cats have a significantly shorter average lifespan than their non-azotaemic counterparts (Williams et al, 2010a).
This contrasts with the situation of a patient whose mild CKD only becomes apparent after hyperthyroidism is treated; these patients do not have a shortened survival time compared to non-azotaemic, well-controlled hyperthyroids.
This is important given various studies demonstrate that a large number of cats (between 15% and 50%) can be expected to become azotaemic during the first few months after restoration of euthyroidism. This is usually well-tolerated by the patient. As creatinine levels are typically lowered by the loss of muscle mass in thin hyperthyroid cats, and polydipsia/polyuria in hyperthyroidism can contribute to dilute urine, a patient’s true renal function may not be fully appreciated until euthyroidism is achieved.
A study by Peterson et al (2018) showed that symmetric dimethylarginine measurement (SDMA) could sometimes be useful in predicting which cats may become azotaemic in the months after treatment of hyperthyroidism (often termed “unmasking” of CKD).
To be forewarned is to be forearmed, perhaps, but the findings of a raised SDMA should not necessarily deter the clinician from offering a definitive treatment as other studies have indicated that long-term suboptimal control of hyperthyroidism (as is typically achieved with medical management) has a further detrimental effect on the kidneys.
As our knowledge stands, currently the recommendation would be to treat a hyperthyroid cat even if mild azotaemia was to occur following restoration of the euthyroid state.
Hyperthyroidism can be cured by surgery or radioactive iodine treatment. Alternatively, long-term medical management is possible using anti-thyroid drugs.
An iodine-restricted diet is available – this is rarely an optimal treatment modality long term, although it is sometimes used this way. The literature cites other possible treatments (for example, ethanol ablation, short-term use of sodium ipodate), but these are rarely used.
Treatment should be carefully selected on a case-by-case basis rather than relying on a standard protocol. Wherever possible, a curative treatment should be the aim, particularly in the younger age categories.
Compliance with anti-thyroid medication may decline over time, leading to cardiac and renal complications of hyperthyroidism.
Of equal or greater concern, the chance of malignant transformation of adenomatous thyroid tissue is measurable and increases with time (Peterson et al, 2016). Should this occur, curative treatment of the hyperthyroidism and malignancy is limited; an opportunity has been missed and a poorer prognosis permitted.
Anti-thyroid drugs are often used short term to assess the renal effects of restoration of a euthyroid state prior to pursuing a definitive treatment, and/or to achieve better cardiovascular stability prior to general anaesthetic for surgical thyroidectomy.
Additionally, thiamazole or carbimazole (which is metabolised to thiamazole), are often employed to control the hyperthyroid state long-term. These drugs interfere with the synthesis of thyroid hormones by inhibiting the enzyme that incorporates iodine. They do nothing to prevent the growth of the adenomatous tissue, and ongoing thyroid gland growth will demand an ever-increasing dose of medication over time to maintain euthyroidism.
A persistently raised T4 may indicate poor owner or patient compliance, an inadequate dose of medication, or rapid metabolism of the drug – particularly where once-a-day administration of thiamazole is being used and the blood sample is drawn 12 to 24 hours or more after the tablet was last administered.
The occasional cat requires a very high dose of anti-thyroid medication to achieve adequate control. As long as side effects do not develop, the dose can be increased as required, even above the data-sheet dose range if needed, under the cascade.
When monitoring response to therapy, it is desirable for the TT4 to sit in the lower half of the deference range. Avoiding levels higher than this ensures that control remains adequate despite the daily fluctuations of thyroxine expected in all patients.
However, debate exists around the optimal target TT4 for treated hyperthyroid cats, fuelled by the recognition that (iatrogenic) hypothyroidism can be associated with the development of azotaemia, and this combination typically leads to a shortened survival time (reduced by about half; Williams et al, 2010b).
In this study, cTSH was elevated in nearly 70%, implying genuine hypothyroidism rather than non-thyroidal illness.
It is accepted that the lower-mid reference range is where the total T4 should ideally sit, regardless of treatment modality used (Daminet et al, 2014). It is less clear what long-term effect a lower, but still normal TT4 might have on glomerular filtration rate and survival.
The narrow target of the lower-mid range can be difficult to achieve, and striving rigidly for this aim may lead to a frustrating situation where the TT4 “yo-yos” between too high and too low. In some cases, it may be better to accept a TT4 well into the lower half of (but still within) the reference range – if the patient is not azotaemic – in favour of losing owner compliance with dose adjustments and monitoring tests. The impact on the cat of repeated visits to monitor the effect of repeated dose changes also must be taken into account.
Conversely, if azotaemia is occurring when the TT4 drops lower than ideal, it can be helpful to use a liquid anti-thyroid medication where the dose can be more finely titrated. Another situation that can present a diagnostic and treatment challenge is a patient whose TT4 and renal values have been stable for some time when a monitoring test unexpectedly shows azotaemia and a low TT4. Although iatrogenic hypothyroidism may be evident, bear in mind also that concurrent advancing CKD can act as a non-thyroidal illness that suppresses TT4.
Urinalysis (specifically for concentrating ability), and TSH measurement, can help determine which is cause and which is effect in an azotaemic and apparently hypothyroid cat (Aldridge et al, 2015).
Direct side effects of anti-thyroid medications are varied. Some can be mild and self-limiting, such as lethargy, vomiting and anorexia; where this occurs, usually temporarily lowering the dose and ensuring administration is with food is enough to resolve the signs. Adverse effects, other than these intestinal effects, will generally recur whenever and however the drug is re-introduced. Hepatotoxicity is well recognised, with hepatic necrosis and intrahepatic cholestasis causing jaundice. This is reversible once the drug is withdrawn.
Blood dyscrasias are rarer, but well-recognised side effects of anti-thyroid drugs. These are frequently mild and of little significance (for example, eosinophilia, lymphocytosis). However, some abnormalities are more severe and require cessation of the drug; of these, leukopenia and particularly neutropenia is seen most commonly.
Haemolytic anaemia is reported, and it can be severe, and thrombocytopenia can occur, sometimes to the extent that allows spontaneous haemorrhage.
The occasional cat will show profound excoriation of the skin, usually around the face. The condition responds poorly to symptomatic treatment including steroids and always necessitates withdrawal of the drug, after which resolution of the problem usually occurs, although sometimes frustratingly slowly.
Evidence exists that topical thiamazole is preferred to oral medication by some owners. Studies have confirmed efficacy via this route and with a potentially easier method of administration, compliance would hopefully be improved, but some studies have implied that this is not always the case (Boretti et al, 2014).
The incidence of gastrointestinal side effects is reduced compared to oral anti-thyroid medication, but other side effects occur with the same frequency.
The drug is usually applied on the underside of the pinna, so that oral ingestion by the cat should be minimised. In some patients, local irritation can occur and prior application of a small amount of topical steroid medication can reduce this. Recent studies have indicated that when mixed with some lipophilic carrier molecules, thiamazole may reach the upper side of the ear, which could have some safety implications for owners (Hill et al, 2015).
With any chronic disease requiring daily medication, both owner and patient compliance can wane over time. Various strategies may prevent a decline in enthusiasm or serve to reignite it.
We can assume that a poorly controlled hyperthyroid feline has similar experiences to a hyperthyroid person, and these are unpleasant, ranging from breathlessness and palpitations, to anxiety and irritability. This is not anthropomorphising, but empathising across the species boundary.
Owners should be given information on all the possible treatment modalities available and involved in the decision-making process.
When considering long-term medical management, owners should be counselled at the outset that a schedule of daily, often twice-daily administration of medication must be adhered to; regular monitoring is essential; and dose adjustments are to be expected. Long-term, costs will mount, and clients should be prepared for this. If compliance with medication is erratic or consistently poor, it may be an indication to reconsider a definitive treatment modality.
Some of steps that professionals can take to assist and advise owners with medicating at home include:

Some barriers to monitoring that professionals must consider include:
The surgical technique for thyroidectomy is not especially demanding; however, tissue handling is important, as inadvertent damage to local structures can result in significant post-surgical morbidity (including Horner’s, laryngeal paralysis, hypocalcaemia).
Hypothyroidism was considered an uncommon consequence of thyroidectomy, even bilateral surgery. This is presumably because tiny amounts of normal, atrophied thyroid tissue are left behind, and this regenerates and recovers function over time.
However, as cats typically show so few signs relating to hypothyroidism, it is possible that hypothyroidism following surgical thyroidectomy is more common than initially appreciated. A recent study found 17% of 81 cats remained hypothyroid long-term following thyroidectomy, and others were lost to follow-up (Covey et al, 2019). TT4 should be monitored proactively, so if persistent hypothyroidism is identified, thyroxine supplementation can be instituted.
When a cat undergoes a unilateral thyroidectomy, in around 70% of cases the second gland does become over-active in time. Additionally, a problem that has become increasingly recognised is the presence of ectopic, usually intra-thoracic thyroid tissue. This can lead to a failure of a bilateral thyroidectomy to achieve resolution of hyperthyroidism. Such tissue can be removed surgically if the clinician is aware of its presence, and a thoracotomy is not always required if the mass can be reached from the thoracic inlet.
Scintigraphy would be the ideal way to assess every hyperthyroid cat preoperatively, but access to this technique is limited. Medical management or radioactive iodine treatment are suitable alternative therapies for cats with ectopic thyroid tissue.
Radioactive iodine (RI) treatment requires no general anaesthetic, is effective against ectopic thyroid tissue, and there is no risk of post-treatment hypocalcaemia.
Response rates are very good, with a cure achieved in 95% of cases after a single injection; a further 2% to 5% of cases respond to a second injection.
Late recurrence of hyperthyroidism is only very rarely seen following RI therapy; the treatment includes any subclinically active adenomatous tissue, which is presumably a cause of recurrence of hyperthyroidism some months following a unilateral surgery.
Adenomatous tissue actively concentrates the radioactive iodine, which emits gamma and beta radiation, causing cell necrosis within a very small range (2mm), and sparing the parathyroid glands. Normal thyroid tissue has atrophied and does not take up the iodine efficiently, so it is also relatively protected.
The true incidence of hypothyroidism following radioiodine treatment has been the subject of much study in recent years. Many cats may show biochemical hypothyroidism, as evidenced by a sub-normal TT4 measurement; however, most patients show no signs of hypothyroidism, potentially because T3 levels are maintained, although this has not been well-studied.
However, as already discussed, an association between the development of hypothyroidism and azotaemia, and subsequent shortened survival times, has been demonstrated and this would preferably be avoided if possible. If this situation occurs, supplementation of thyroid hormone is advised, although it is still a matter of opinion when this should be commenced, given recovery of function of remaining normal thyroid tissue may occur over several (usually three to six) months following treatment.
FT4 and cTSH measurements may be of assistance in decision making, and any compromise to renal function should prompt more aggressive intervention. Late development of hypothyroidism has been documented unexpectedly and only rarely.
Thyroid carcinomas are found in around 3% of hyperthyroid feline patients. Surgical removal can be attempted, although they are typically locally invasive and sometimes metastasise, usually to local lymph nodes, and usually the best that can be achieved is a de-bulking effect.
This provides histopathologic confirmation of the malignancy and may lower the dose of anti-thyroid medication that is required to control clinical signs of hyperthyroidism, which makes management of the condition easier.
Alternatively, high-dose radioactive iodine treatment (5 times to 10 times the conventional dose given to cats with an adenoma) can be given in addition to surgery with curative intent, usually with good results.
To the author’s knowledge, the universities of Bristol and Glasgow remain the only centres in the UK to carry a licence permitting the use of high-dose RI treatment. It is encouraging to note that a small study from Bristol showed encouraging results of RI treatment as the single treatment modality for thyroid carcinoma, with six out of eight cats cured and having lengthy survival times (Hibbert et al, 2009).
Hill’s y/d diet is a prescription food available in the UK for the treatment of hyperthyroid cats with benign disease.
The diet is depleted in iodine, depriving the thyroid gland of the element it requires to synthesise the thyroid hormones. Hill’s y/d is marketed to restore the euthyroid state in hyperthyroid cats, either as a sole treatment or in conjunction with anti-thyroid drugs. Because of the thyroid’s ability to concentrate any consumed iodine, the diet should be fed as the sole food source to maintain its effect. It is, therefore, potentially unsuitable for cats with outdoor access, and it may be difficult to regulate feeding in multi-cat households.
Small studies carried out by Hill’s did show good results with dietary iodine restriction, varying from partial to complete control, although it should be noted that to reliably establish euthyroidism with a diet containing iodine levels equivalent to that of Hills y/d (0.32 parts per million), it seems the cats in the study had access only to de-ionised water.
Anecdotally, in the clinical setting, results seem to vary from good in hyperthyroid cats with mild-to-moderate elevations in TT4 and where the diet is well accepted as a sole food source, to disappointing where compliance cannot be maintained or hyperthyroidism is more severe.
The diet is not considered suitable for long-term treatment, at least in part because it contains insufficient protein levels, leading to weight loss and sarcopenia in particular. Other signs such as tachycardia are also reported to be persistent, even if the TT4 normalises (Hui et al, 2015).
Diagnosis of hyperthyroidism is often, but not always, straight-forward, and a range of thyroid function tests may be required in some cases. Individual patient and owner factors should be considered when selecting the most appropriate treatment modality for each case.
Anti-thyroid drugs can be used long-term, but treatment must be monitored and compliance ensured.