20 May 2022

Image © guruXOX / Adobe Stock

Cats can be exposed to many toxic substances – and ethylene glycol (EG) remains one of the most common2.
EG – also known as ethanediol – is an odourless liquid that is clear and sweet in taste, making it attractive to animals. It is used in antifreeze solutions in many motorised vehicles, coolants, rust removal solutions, printer ink3, as a preservative for taxidermy4 and as a chemical intermediary for some plastics3. The concentration of EG can vary significantly, but can be up to 100% in some products3.
Animals are commonly exposed to EG from vehicles leaking the substance on to the ground, where it can be ingested. Even the smallest spill poses a real threat to small animals, especially cats. Any amount ingested by cats requires immediate treatment. The availability of this substance – along with a low minimum lethal dose and a lack of public knowledge – plays a large role in the frequency of toxicity.
Some products have been replaced with a less toxic formulation, propylene glycol2. This is less palatable and converted into lactic acid within the body. Bittering agents may occasionally be used to help deter ingestion3.
EG is not initially toxic, but as it is metabolised by the body, the by-product metabolites cause toxic side effects.
Once a patient has ingested EG, it undergoes rapid gastrointestinal absorption before being metabolised by the liver and excreted renally. Early CNS signs may develop due to the unmetabolised EG3. Toxic metabolites called glycolic acid are formed as a by-product, which cause severe metabolic acidosis, renal and cardiopulmonary signs.
Clinical signs and laboratory changes 30 minutes to 12 hours after exposure include:
Laboratory changes include acidaemia, hypocalcaemia may start to develop, electrolyte disturbances, and increased serum osmolality leading to an osmotic diuresis.
Clinical signs and laboratory changes 12 to 24 hours after exposure include:
Laboratory changes include proteinuria, haematuria, glycosuria and albuminuria, and calcium oxalate crystals may be present in the urine. Urine specific gravity (USG) is likely to decrease, but remain above the isosthenuric range. Urinary pH typically remains low (lower than pH6). Hyperglycaemia and ionised hypocalcaemia are seen in more than 50% of cases2. Azotaemia and hyperphosphataemia will develop as the acute kidney injury (AKI) progresses2. Electrolyte and acid base disturbances are also exacerbated by renal impairment3.
Serum calcium combines with the metabolite oxalic acid, which leads to the formation of calcium oxalate crystals within the vascular system and kidneys2. The crystals become deposited in the renal tubules, leading to a life-threatening AKI.
AKI is defined as an abrupt decrease in renal function, which may include both structural and functional damage. Azotaemia occurs due to a decrease in the excretion of nitrogenous waste products (urea, creatinine and phosphate). Electrolyte abnormalities – such as hyperkalaemia and metabolic acidosis – are likely.
AKI is often not a syndrome of one organ, but a systemic illness causing multiple organ dysfunction syndrome.
The three AKI phases to the development of clinical signs4 are detailed in Figure 1.
Diagnosis is very rarely confirmed with laboratory analysis and is based on suspicion of exposure, combined with clinical signs and exclusion of other causes3. Exposure to EG is strongly suggestive if calcium oxalate formation (renal mineralisation and urolithiasis) are present5, along with severe metabolic acidosis, decreased urinary output and AKI6.
EG test kits can detect blood levels within 30 minutes of ingestion. The kits are also sensitive to propylene glycol and glycerol, which can provide a false-positive result. They carry a relatively high detection limit of greater than 50mg/dl. Cats can be intoxicated with a much lower limit of 20mg/dl – meaning exposed cats can have a negative result4.
EG concentrations are only detectable in urine or serum between 48 hours and 72 hours post-ingestion, which is often too late to initiate life-saving therapy4.
Fluorescein is often added to EG to help identify radiator leaks. This can often be detected with UV light in the urine of affected animals.
Fluorescein has a half-life of slightly more than four hours and can only be detected in urine with a pH lower than 4.5.
A Wood’s lamp can prove useful to check for fluorescein in the mouth and vomit in the emergency setting.
Some urine collection bags have a fluorescent lining, which could lead to a false-positive result7. This technique carries limitations and should not be used as a definitive diagnostic method; however, it can be a useful bedside technique.
EG is rapidly absorbed into the gastrointestinal tract; therefore, inducing emesis or nasogastric aspiration should only be considered within one hour of ingestion. It does not readily bind to activated charcoal, so administration is not advised2.
A treatment plan should be formed around the prevention of toxic metabolite conversion, reversal of electrolyte and acid base disturbances, and maintaining renal function. IV fluid therapy should be administered along with an antidote. Both ethanol and fomepizole act directly on alcohol dehydrogenase. This prevents metabolisation of toxic metabolites, allowing unmetabolised EG to be excreted renally. Fomepizole is more effective and safer to use than ethanol1. Fomepizole is very expensive and cats require around six times the dose in comparison to dogs8.
Time plays a vital role in the successful treatment of this toxicity and cats must start treatment within three hours of ingestion2. Treatment initiated beyond this time is often associated with a mortality rate of almost 100%2.
It is important to administer ethanol for at least 48 hours to block metabolism of EG and allow renal excretion.
Ethanol often causes depression of the CNS and worsening of metabolic acidosis may be seen. Fewer side effects are often associated with fomepizole, and it is deemed a safer and more effective drug.
Other forms of alcohol often contain other substances, making them unsafe for IV administration. The least dangerous type of commercially available alcohol is likely to be vodka, which can be administered orally via a naso-oesophageal feeding tube for clinically mild cases1. If oral administration is not possible, a solution containing 40% vodka can be administered IV8.
Administration of antidote therapy is contraindicated in cats once renal injury is present3.
Haemodialysis is available at some hospitals in the UK and may be beneficial for the removal of ethylene glycol and its harmful metabolites2. This is often started prior to the onset of an AKI. EG is readily dialysable, although facilities are limited to a small number of veterinary hospitals across the UK.
Nurses are heavily involved in the direct care of the AKI patient in an intensive care setting. The intensity of these cases ideally requires one-to-one nursing care in a 24-hour hospital.
The following requirements are vital for the treatment of AKI:
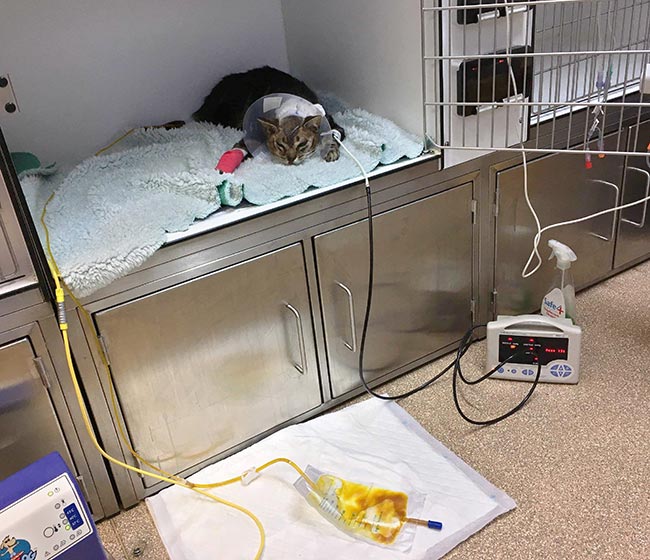
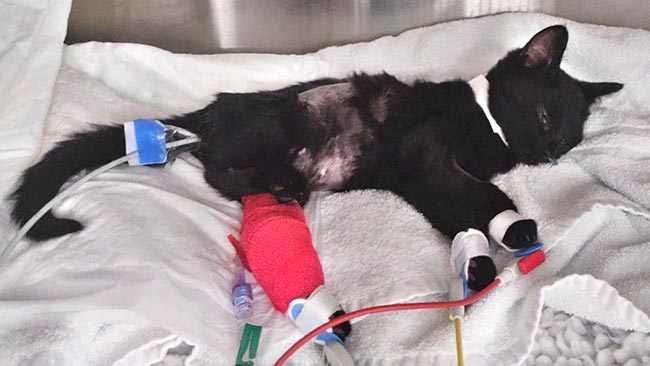
Ethylene glycol toxicity carries a very serious risk to cats. Unless exposure to the toxin was witnessed, cats often present during the later stage of poisoning, which limits treatment options.
According to the Veterinary Poisons Information Service, the overall mortality rate of cats with a confirmed or suspected diagnosis to EG is 85%3. Given that very few cases are confirmed as EG toxicity, it is possible that the recovering cases had only suspected EG exposure.