10 Sept 2018

Idiopathic dilated cardiomyopathy (DCM) is a disease of the heart muscle characterised by cardiac chamber dilation, and systolic dysfunction with or without tachyarrhythmias (Figure 1). It is a diagnosis of exclusion that requires echocardiography for accurate assessment, although some ancillary tests can increase the clinician’s index of suspicion.
Dogs with DCM eventually develop congestive heart failure (CHF) and forward output failure, at which point quadruple therapy – consisting of furosemide, pimobendan, an angiotensin-converting enzyme (ACE) inhibitor and spironolactone – is recommended.
Haemodynamically significant arrhythmias can be a feature of DCM in a number of breeds, and assessment with Holter ECG monitoring prior to antiarrhythmic therapy is recommended.
Treatment of preclinical (also termed occult or asymptomatic) DCM is a relatively new advancement in veterinary cardiology, and studies have identified a survival benefit for both an ACE inhibitor and pimobendan, with extension of the preclinical phase as well as improved survival.
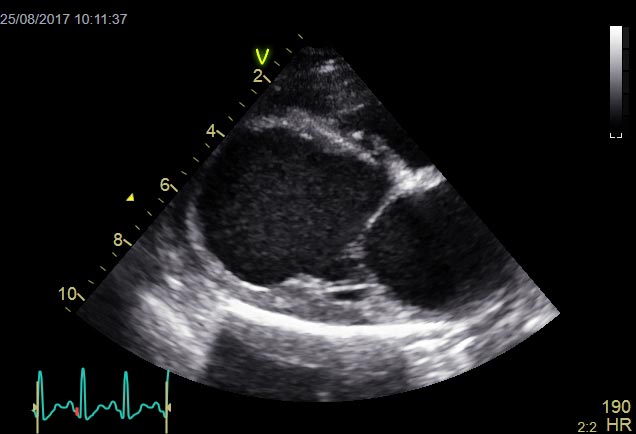
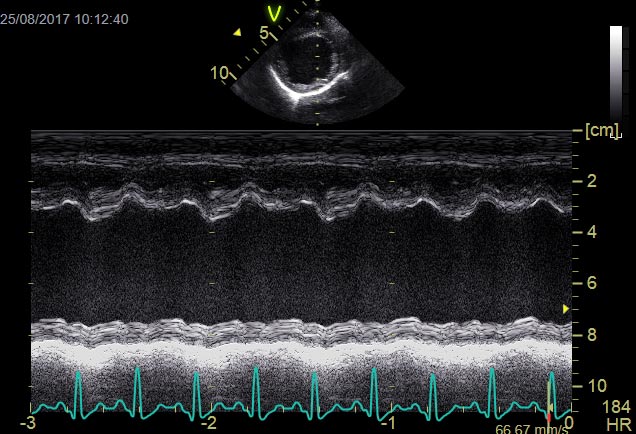
In human DCM, causes include viral, autoimmune, alcoholic and toxic injury, with only between 30% and 50% of cases considered hereditary. In contrast, canine DCM in some breeds reaches a prevalence of 60%1 and the majority of cases are considered genetic in origin.
DCM is typically a disease of large and giant breeds – most notably the boxer, dogue de Bordeaux, Dobermann, great Dane, Newfoundland, deerhound, Irish wolfhound, Labrador retriever and St Bernard – and is uncommonly noted in cross-breeds. DCM has also been reported in smaller breeds, including the English cocker spaniel, American cocker spaniel, springer spaniel and Portuguese water dog, and a family of Manchester terriers.
While a genetic basis is considered highly likely across a large number of breeds, other causes have included taurine deficiency and L-carnitine deficiency. Similarly, links have been established with hypothyroidism; however, while systolic function can improve with treatment, no significant reverse remodeling occurs2.
The prevalence of DCM can be as high as between 50% and 60% in some breeds1,3, with important differences in disease manifestation between males and females. For example, male Dobermanns show a systolic dysfunction phenotype, whereas female Dobermanns tend to have a higher risk of ventricular arrhythmias.
Importantly, idiopathic DCM is a diagnosis of exclusion, so both acquired and congenital cardiac disease – in addition to systemic non-cardiac disease – must all be excluded. Dilation of the left ventricle, with impaired systolic function, may be seen secondary to both congenital and acquired heart diseases – including patent ductus arteriosus, mitral valve dysplasia, end-stage mitral valve disease and sustained tachyarrhythmias – so assessment should always include detailed echocardiographic assessment.
Generally, canine DCM has a long preclinical period, although will invariably lead to the development of heart failure, arrhythmias and/or sudden cardiac death (SCD).
Assessment during the preclinical phase can be challenging and requires echocardiography, as well as Holter ECG in certain breeds where arrhythmias may be an early feature of the disease course.
Echocardiographic changes can include a combination of left ventricular dilation in both end-systole and end-diastole, as well as reduced fractional shortening, ejection fraction and increased E-point septal separation (EPSS)4; although in preclinical DCM these may be absent or equivocal, leading to real difficulties making an accurate diagnosis.
Interestingly, while these echocardiographic abnormalities allow for a diagnosis of DCM, they are poor correlates of clinical signs, exercise capacity and life expectancy. More advanced indices – such as end-systolic volume index greater than 140ml/m2, severity of heart failure, the presence of ascites, severe reduction of ejection fraction (lower than 25%) and advanced diastolic dysfunction (restrictive transmitral flow pattern) – are important negative prognostic indicators5.
An important adjunctive diagnostic test is ECG Holter monitoring with a 24-hour recording, because it has been shown some breeds – such as the Dobermann, great Dane and boxer – can have severe tachyarrhythmias, including both ventricular and supraventricular tachycardias.
Screening tests – including N-terminal pro-brain natriuretic peptide (NT-proBNP) and cardiac troponin I – also have a place in the diagnosis of DCM, but positive and equivocal results always necessitate follow-up with diagnostic imaging.
Doppler echocardiography is considered the gold standard tool for detailed evaluation in veterinary medicine; advanced imaging modalities – such as cardiac MRI – are more sensitive in humans, but remain challenging in the conscious dog.
The biomarker NT-proBNP, an indicator of myocardial stretch, has been shown to be a useful screening tool for “at-risk” breeds and dogs with murmurs. Generally, an NT-proBNP lower than 900pmol/L is considered less compatible with significant cardiac disease, although breed variations need to be taken into consideration when interpreting values6. An even more sensitive test in Dobermanns for screening for occult DCM is the combination of NT-proBNP values with a Holter ECG7.
NT-proBNP values greater than 900pmol/L in a dog with a murmur suggests increased stretch and stress on the myocardium, and supports the presence of clinically significant heart disease. Of importance is the test is neither 100% sensitive or specific – so if a strong index of suspicion already exists, echocardiography may be more appropriate than NT-proBNP for diagnosis, severity assessment, treatment decisions and prognosis.
Cardiac troponin I, another useful biomarker, is an indicator of cardiomyocyte damage and has been shown to be elevated in DCM in a number of breeds8. Values greater than 0.22ng/ml in the Dobermann has been shown to be strongly correlated with DCM8, even in apparently healthy animals, although echocardiography is required to stratify severity. Evidence also exists for its use in predicting the risk of SCD, in addition to a prognostic value of the test.
A number of diagnostic tests may increase the index of suspicion for clinicians; however, at this time, echocardiography is the gold standard for determining the severity of DCM (with or without Holter ECG monitoring, depending on the breed) and allows for appropriate decision-making with regards to medical therapy.
Once a diagnosis of DCM has been established, treatments can be split into those recommended during the preclinical phase or the clinical phase.
DCM results in a reduction in cardiac output that stimulates compensatory mechanisms, including the sympathetic nervous system and renin-angiotensin-aldosterone system (RAAS) – both aim to improve cardiac output and maintain blood pressure. These initially compensatory mechanisms eventually become maldaptive, resulting in CHF and/or forward heart failure.
Treatment of CHF is aimed at addressing and controlling pulmonary oedema, while forward heart failure is addressed with medications that support systolic function and cardiac output.
Diuretics are the essential cornerstone therapy for CHF and relieve pulmonary oedema. The loop diuretics are the most important diuretic and have a high ceiling effect – the higher the dose, the more potent the effect.
Furosemide is the most common loop diuretic used in veterinary medicine, with a predictable response pattern9. If furosemide is administered IV, additional venodilatory effects may occur (for example, offloading of the heart and the pulmonary tree, and further relieving pulmonary congestion), so the IV route can be useful for acutely dyspnoeic animals.
In acute CHF, frequent, low-dose boluses (1mg/kg to 2mg/kg) are administered as often as required – typically every one hour to two hours in the acute setting – and provide rapid diuresis without a precipitous drop in preload, which can be seen with high bolus doses (6mg/kg to 8mg/kg). These high doses, given as a single bolus, may worsen clinical status and are no longer recommended.
Renal function and electrolyte assay should be monitored regularly, particularly after any dose adjustment and/or when high doses of furosemide are administered, and ideally pretreatment where safe to blood sample. The author recommends assessment of blood work between one week and two weeks after furosemide dose increase or adjustment.
Torasemide is an alternative to furosemide and is frequently used in refractory CHF or where furosemide effect is no longer sufficient – for example, where furosemide resistance is suspected. Additional diuretics may become useful as response to loop diuretics begins to diminish; a hydrochlorothiazide/amiloride combination is an adjunctive therapy alongside the loop diuretic that may improve control of CHF and help management of cases with scites secondary to right-sided or biventricular CHF.
It remains best practice to administer an ACE inhibitor alongside a diuretic10. The RAAS is stimulated by volume depletion, which is secondary to diuresis. ACE inhibitors help counteract the deleterious effects of the RAAS in CHF – namely vasoconstriction, and sodium and water retention. ACE inhibitors should, therefore, be administered alongside a diuretic for a dog in CHF to counteract the RAAS. ACE inhibitors available in the UK are included in Table 1.
| Table 1. Drugs and doses for the treatment of congestive heart failure (CHF) | |||
|---|---|---|---|
| Drug | Dose | Frequency | Notes |
| Furosemide | Acute: 1mg/kg to 2mg/kg boluses (can give up to 4mg/kg bolus where necessary) IV | Every 30 minutes if required initially, aiming to slowly reduce frequency to every 4 to 6 hours as respiratory rate decreases | Monitor electrolytes and renal parameters. |
| Chronic: 1mg/kg to 4mg/kg orally | Every 8 to 12 hours | Consider additional or alternative diuretics when furosemide dose exceeds 8mg/kg/day to 10mg/kg/day | |
| Torasemide | 0.1mg/kg to 0.6mg/kg orally | Once daily (twice a day off-licence) | Licensed for stable CHF. Close monitoring of electrolytes and renal parameters recommended |
| Hydrochlorthiazide/amiloride | 0.5mg/kg to 2mg/kg orally | Once daily to twice a day | Gradual increases in doses recommended, with close monitoring of electrolytes where used in combination with a loop diuretic. |
| Benazepril | 0.25mg/kg to 0.5mg/kg orally | Once daily | Based on pharmacokinetic data, may be increased to twice a day dosing in late CHF. |
| Enalapril | 0.5mg/kg orally | Once daily to twice a day | |
| Ramipril | 0.125mg/kg to 0.25mg/kg orally | Once daily | |
| Imidapril | 0.25mg/kg orally | Once daily | Liquid formulation angiotensin-converting enzyme inhibitor. |
| Pimobendan | 0.1mg/kg to 0.3mg/kg orally | Twice a day | May result in transient vomiting and diarrhoea. |
| Spironolactone | Once daily | Monitor potassium, although hyperkalaemia rarely seen to clinically significant levels. | |
ACE inhibitors can result in hypotension due to their vasodilatory properties, so should not be given in the presence of more than mild systemic hypotension (where systolic blood pressure is lower than 90mmHg). ACE inhibitors have been shown to have comparable efficacy, so no preferential ACE inhibitor exists.
Both aldosterone and angiotensin II promote myocardial fibrosis, and ACE inhibitors help to reduce this; however, aldosterone antagonists may also be beneficial to reduce fibrosis. Indeed, it has been shown with mitral valve disease a third of animals will have ongoing RAAS activation where an ACE inhibitor is used in isolation11. Aldosterone antagonists will help reduce aldosterone formation, both in these specific cases and in any dog with aldosterone activation via any method.
Spironolactone is the commonly used veterinary product, and has been shown to reduce mortality in humans and the risk of cardiac-related death in dogs with CHF secondary to mitral valve disease. Aldosterone antagonists, such as spironolactone, are also weak potassium-sparing diuretics, in addition to the previously reported beneficial effects on cardiac remodeling. These effects have not been shown in canine DCM; however, their use is extrapolated from other cardiac conditions causing CHF. As a result, the author uses spironolactone regularly in all canine cases with CHF, including cases secondary to DCM.
Pimobendan is a phosphodiesterase III inhibitor, with positive inotropic and balanced vasodilatory effects. The improved systolic function, coupled with a reduction in afterload, improves cardiac output and clinical signs. Pimobendan is licensed for the management of canine DCM and has been shown to prolong the time to development of refractory pulmonary oedema or death in Dobermanns12.
Historically, digoxin has been used for positive inotropic effects; however, due to its potential proarrhythmic effect, pimobendan is considered a safer and more efficacious positive inotrope; digoxin is now almost exclusively reserved for antiarrhythmic therapy in atrial fibrillation.
In the acute setting, an IV formulation of pimobendan is also available that may be beneficial in recumbent, or poorly responsive, critical patients.
For dogs that present acutely, minimising stress, providing supplemental oxygen and cage rest are also important. For severely compromised patients, other additional options of variable benefit may include nitroglycerine ointment/patches that have variable venodilatory effects and reduce preload. Constant rate infusions of furosemide may also be considered, although debate exists as to whether this route of administration provides additional benefit.
Other intense therapy options include sodium nitroprusside (potent vasodilator) and dobutamine (positive inotrope); however, both treatments require close monitoring in a hospital clinic with blood pressure and ECG monitoring.
Treatment in the preclinical phase has been gaining much attention and strong evidence exists to support the use of pimobendan. Some retrospective data also supports the use of ACE inhibitors, although evidence is limited to a small number of breeds, including the Irish wolfhound and Dobermann. Even so, it is reasonable practice to use on the cascade for other animals, given the benefit in these breeds.
The PROTECT Study13 is one of the largest double-blinded, placebo-controlled veterinary studies to date, which investigated the use of pimobendan versus placebo for treatment of preclinical DCM in Dobermanns. Pimobendan prolonged the preclinical phase and extended survival time by approximately nine months compared to placebo. Additionally, no increased risk of SCD or proarrhythmia was seen in the pimobendan group. The data supported the use of pimobendan in Dobermanns with preclinical DCM, so rationale exists for its use across other large breeds for this reason. Importantly, assessment for the presence of preclinical DCM requires echocardiography with or without 24-hour Holter ECG, the latter dependent on the breed. An ongoing study (SHIELD) is assessing the efficacy of pimobendan in preclinical DCM in other large to giant breeds.
ACE inhibitors have been shown to increase median time to onset of clinical signs in Dobermanns with DCM14, similar to those reported in humans administered ACE inhibitors with impaired systolic function (SOLVD trial). As a result, a small evidence base exists to recommend their use in preclinical DCM in an attempt to prolong the time to onset of CHF.
Historically, beta-blockers have been investigated for preclinical treatment of DCM; however, results were disappointing and their use is not recommended in dogs at this time.
It is important to stress the majority of the studies for preclinical treatment have been performed in Dobermanns and, therefore, treatment in other breeds is extrapolated. Given the stark prognosis for clinical DCM, if treatment in the preclinical phase infers either a delay to the onset of CHF or a survival benefit, it’s the author’s opinion pimobendan use in other breeds can be justified.
Additionally, the use of individual breed normal echocardiographic indices is essential in the diagnosis of DCM, as much variation in systolic function can exist between breeds, particularly in breeds considered to be athletic, such as the border collie, springer spaniel and English cocker spaniel.
Both ventricular and supraventricular arrhythmias can be seen as part of DCM.
Arrhythmias may cause clinical signs, such as exercise intolerance, syncope, tachypnoea and SCD. By using a Holter ECG, the presence of arrhythmias may help reach the diagnosis of DCM and aid clinicians in determining whether antiarrhythmic therapy is appropriate.
It is important to note antiarrhythmic therapy for ventricular arrhythmias has not been shown to improve survival, although it may reduce episodes of clinical signs such as syncope.
Conversely, optimal rate control of atrial fibrillation based on Holter ECG data has been shown to prolong survival. Therefore, it is recommended ventricular rate of atrial fibrillation be controlled, aiming for a 24-hour Holter mean heart rate of lower than 125bpm15.
Treatment of arrhythmias must take into consideration that a number of antiarrhythmics may further depress systolic function and worsen the patient’s clinical status. For this reason, antiarrhythmics should be reserved for only when an ECG diagnosis is confirmed.
Atrial fibrillation (AF) may develop with DCM following atrial stretch and dilation (Figure 2). Treatment is aimed at slowing the ventricular response rate and not re-establishing a sinus rhythm. Optimal rate control of AF has been shown to improve survival and should be actively pursued wherever possible.
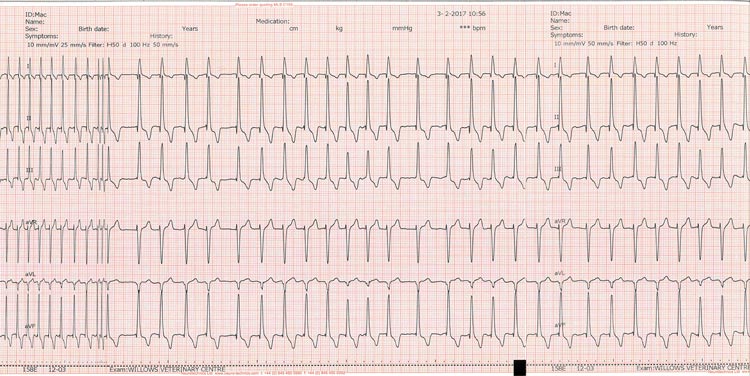
| Table 2. Drugs and doses for the treatment of atrial fibrillation | |||
|---|---|---|---|
| Drug | Dose | Frequency | Notes |
| Digoxin | 0.002mg/kg to 0.005mg/kg | Every 12 hours | • Lower doses appear to be satisfactory with combination treatment. • Lower doses in large breeds may be more appropriate. • Monitoring digoxin levels is recommended wherever possible, or when clinical signs of toxicity are suspected |
| Diltiazem | Modified release preparation: 0.5mg/kg to 2mg/kg | Every 8 hours | Start at 1mg/kg initially and titrate accordingly. Lower doses may be appropriate in the presence of congestive heart failure. |
| Sotalol | 0.5mg/kg to 3mg/kg | Every 12 hours | Lower doses recommended with myocardial dysfunction. |
| Enalapril | Loading schedule: 10mg/kg to 15mg/kg orally every 12 hours for 7 days, then 5mg/kg to 7.5mg/kg orally every 12 hours for 14 days, thereafter 5mg/kg to 7.5mg/kg orally every 24 hours | Regular monitoring for thyroid and hepatic toxicities. Amiodarone levels should be assessed after three weeks of therapy. | |
The drugs and doses for the treatment of AF are listed in Table 2. The recommended therapy of choice for AF in the presence of structural heart disease is a combination of digoxin and diltiazem. The combination has been shown to provide better control of AF than either drug used in isolation16. However, some individual cases respond well to monotherapy.
Diltiazem is a calcium channel blocker and slows conduction through the atrioventricular node, and is useful for controlling supraventricular arrhythmias. Diltiazem has mild negative inotropic effects and may depress systolic function. In the author’s experience negative effects of fast AF on systolic function and cardiac output are more damaging than the mild negative inotropic effect of diltiazem, which appears to be well tolerated by the large majority of AF patients. Diltiazem has a rapid onset of action and slows fast AF quicker than digoxin.
Digoxin is a vagomimetic drug that slows conduction through the AV node and is used to slow supraventricular arrhythmias. It is also a positive inotrope and has historically been used in canine DCM, although is now superseded by pimobendan. It has a narrow therapeutic index and toxicity is frequently seen, especially in large breeds. Digoxin takes approximately one week to reach therapeutic serum levels and should be adjusted once serum trough levels have been assayed. The beneficial vagomimetic effect is often seen at trough levels (6 hours to 8 hours post-pill) of 0.9ng/ml to 1.2ng/ml, and higher doses tend to result in side effects.
Adjustment on doses of either digoxin or diltiazem should only be performed if the heart rates are still fast – higher than 160bpm in the clinic or higher than 125bpm (mean heart rate) on a 24-hour ECG recording. Adjustments of digoxin should also only be made if the serum trough levels are greater than 1.2ng/ml and/or in the presence of adverse clinical signs.
Other treatment options may include beta-blockers, although these should be used with caution in CHF patients as they may depress systolic function further and cause a patient to decompensate. Amiodarone is another effective antiarrhythmic, but requires close patient monitoring for side effects17.
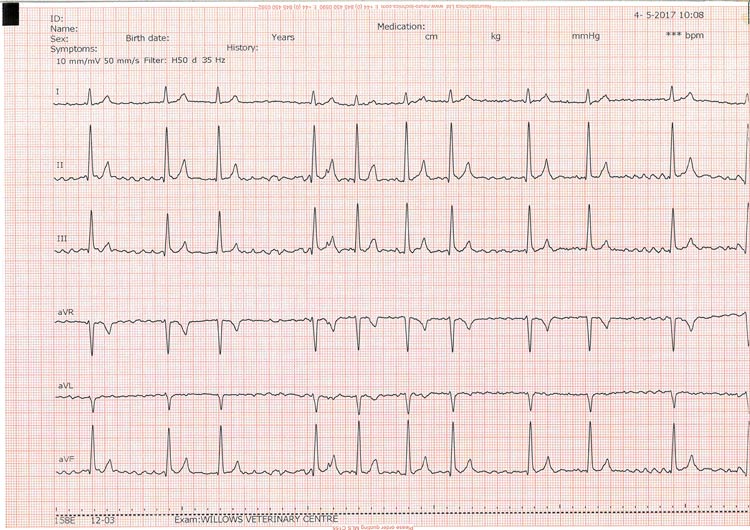
Ventricular arrhythmias can be challenging to diagnose in the clinic, unless one is fortuitous (Figure 3). Even so, a 24-hour Holter ECG should be recommended to assess frequency and severity, or complexity, of ventricular rhythms.
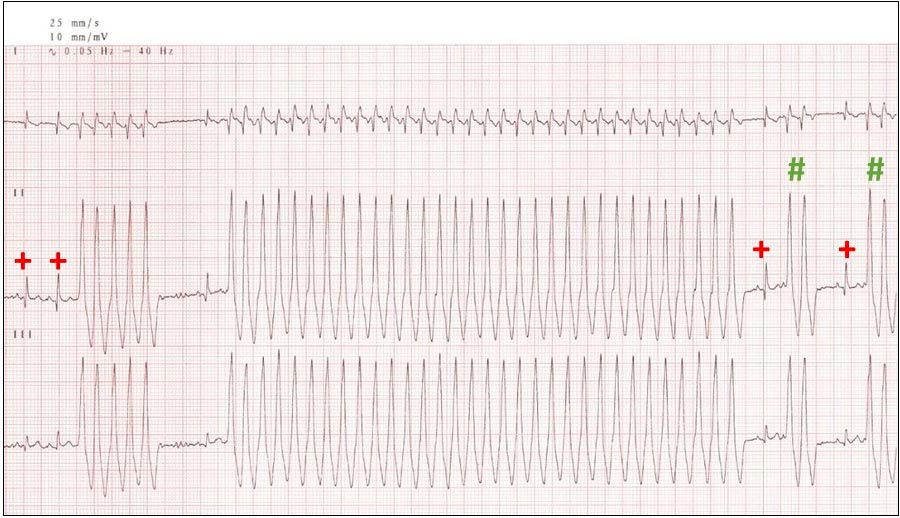
A normal number of ventricular premature contractions (VPCs) may be up to 25 in a 24-hour period. The severity of arrhythmias is based on overall number (more than 1,000 VPCs is considered significant, although not necessarily significant to warrant treatment), complexity (presence of triplets, couplets and runs), the presence of ventricular tachycardia or torsades de pointes, and the presence of clinical signs during a period of ventricular rhythm. Importantly, no evidence exists that treating ventricular arrhythmias reduces the risk of SCD.
The common go-to treatment used in an emergent situation for ventricular tachycardia is lidocaine (a class Ib anti-arrhythmic), which can be administered IV as a bolus (as detailed in Table 1) and as a constant rate infusion if required for sustained ventricular tachycardia or during a crossover on to oral therapy.
Sotalol has mixed antiarrhythmic effects and is less of a negative inotrope than other beta-blockers. It also appears to be tolerated well in patients with systolic dysfunction. Other beta-blockers are usually less favoured due to more significant depression of systolic function and the potential to cause decompensation of previously controlled CHF.
Mexiletine is now available in the UK and can be used as an oral substitute for lidocaine; both are class Ib antiarrhythmics. Mexiletine combined with sotalol, both at standard doses have been shown to be superior to either drug alone in young German shepherd dogs with ventricular arrhythmias and is an option for poorly controlled ventricular arrhythmias in other breeds18.
Amiodarone is another consideration but care should be exercised and patients monitored closely for side effects19.
Omega-3 fish oil supplementation has been shown to reduce cardiac cachexia in clinical DCM and improve appetite in some cardiac patients20. The concentration of omega-3 fish oils shown to provide these responses is best achieved with a veterinary product. Supplementation of taurine and L-carnitine are also considerations if serum levels are low.
Even with prompt medical therapy, the prognosis for DCM with CHF is guarded and breed-dependent. Only about 40% of dogs survive one year after diagnosis21. Earlier treatment during the preclinical phase has been shown to prolong survival and time to CHF in some breeds; therefore, it is recommended wherever possible.
Negative prognostic indicators include a more severe heart failure class, the presence of pulmonary oedema, the presence of VPCs and atrial fibrillation, and a number of echocardiographic parameters. For breeds such as the Dobermann and great Dane, survival times are much shorter than other breeds.
For humans, mechanical ventricular assist pumps, heart transplant and gene therapy are more advanced treatment options, although remain less feasible and unavailable in canine patients at this time.
With such high disease prevalence in certain breeds, strict breed screening and, eventually, regular genetic screening may help reduce the incidence on DCM in the canine population. Until such a time, therapeutic management remains the mainstay treatment. Introducing medical therapy early is demonstrated to improve survival for preclinical DCM, while optimal management of clinical DCM can improve patient quality of life and survival.