18 Dec 2017

The skin is the largest organ of the body and, as most dermatologists would argue, one of the most complex. It is a very reactive organ and can be a source of valuable information, especially as a marker for systemic or internal disease.
The skin is also a very frequent location for primary or metastatic neoplastic disease. It is the most common site of neoplasia in the dog, contributing to one-third of reported tumours, and the second most common in the cat, with a quarter of tumours (Blackwood, 2011). Although mast cell tumours, squamous cell carcinomas and benign cutaneous histiocytomas are often diagnosed – and, therefore, are the most easily recognised cutaneous neoplasms – one form of cutaneous neoplasia is often overlooked: cutaneous lymphoma.
Cutaneous lymphoma is well documented in both dogs and cats, but represents 1% of canine skin tumours (Fontaine et al, 2010). These neoplasms can be divided into two main categories: epitheliotropic lymphoma (EL) and non-EL. EL is a subset of T-cell lymphomas. Cutaneous epitheliotropic T-cell lymphoma is characterised by the presence of neoplastic T-lymphocytes that show a tropism for the epidermis and adnexal structures.
Cutaneous epitheliotropic T-cell lymphoma can be further classified into mycosis fungoides (MF), Sézary syndrome (SS) and pagetoid reticulosis (PR; Miller et al, 2013). Non-EL is less common and can be either B or T large-cell lymphomas of the dermis or SC tissue (Komori et al, 2005), and is often part of a more generalised disease process. While non-EL is more common in the cat, EL is more common in the dog (Moore et al, 1994).
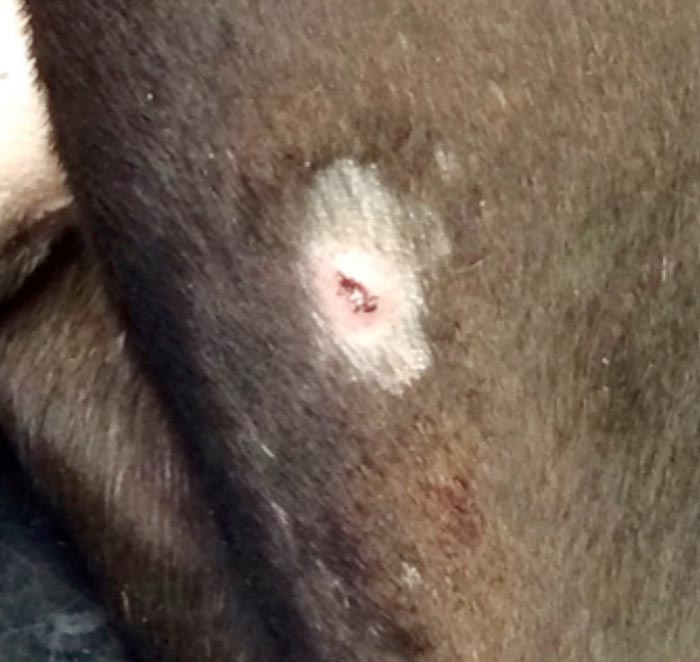
Cutaneous lymphoma is usually either generalised or multifocal. Clinical signs differ slightly between the epitheliotropic and nonepitheliotropic forms, with diagnosis possible only after biopsy and histopathology.
Non-EL occurs in older dogs and cats with no sex predilection. This usually presents as alopecic, erythematous nodules with a multifocal or generalised distribution. Rarely are single nodules seen. Pruritus and oral mucosal involvement is rare (Miller et al, 2013). Unfortunately, the disease course is often short, as lesions progress rapidly and systemic involvement, including lymphadenopathy, is present (Blackwood, 2011).
EL has a slower clinical progression than non-EL. Initially, it has a similar appearance to many other dermatological conditions and is often mistaken for allergic or autoimmune disease (Miller et al, 2013). EL can, however, progress to systemic dissemination. This disease usually affects older dogs and cats, and a predilection may exist in cocker spaniels and boxers (Day, 1995; Moore et al, 1994). Scaling and exfoliative areas are often present and patients are often pruritic.
The clinical course of the cutaneous form or EL can often be divided into three separate stages:
Other clinical signs include mucocutaneous erythema and depigmentation. In a review of 30 cases, 26% of dogs were found to have footpad signs, including hyperkeratosis, ulceration and depigmentation (Fontaine et al, 2010).
EL dogs can be divided into three subforms:
Cats present with similar cutaneous lesions, but not mucocutaneous involvement and are non-pruritic. Lesions are often mistaken as eosinophilic plaques (Fontaine et al, 2011). Advanced cases can also have systemic involvement.
Differentials include superficial pyoderma, atopic dermatitis, ectoparasites (including demodicosis, sarcoptic mange and hookworm dermatitis), autoimmune disease (such as pemphigus vulgaris, bullous pemphigoid and lupus erythematosus), dermatophytosis or Malassezia dermatitis, and eosinophilic plaques in cats.

Diagnosis is best performed early in the course of the disease as the median time between onset of lesions. Diagnosis is 5 months and can span from 0 to 12 months (Fontaine et al, 2010).
While cytology from fine needle aspiration of a cutaneous nodule or an impression smear of any erosive or ulcerated lesions may reveal “round cells” suggesting a lymphoid neoplasm, the final diagnosis of cutaneous lymphoma should always be made on histopathological examination of skin specimens.
Ideally, early lesions – such as erythroderma and exfoliative lesions, as well as nodules – should be sampled by taking elliptical biopsies or using a 6mm to 8mm biopsy punch. It is imperative to take multiple biopsies. These can be performed using sedation and local anaesthesia in the majority of patients with truncal lesions. Those with facial or footpad-only signs should be anaesthetised for biopsy.
Ulcerated areas will be more difficult to interpret due to the loss of the epidermis and are often complicated by secondary bacterial infection. If there is cytological evidence of infection, a section of one sample should also be submitted for bacterial and fungal culture. Samples should be submitted to a dermatohistopathologist for interpretation. As always, a good clinical history should accompany samples. This not only allows the laboratory staff to sample the appropriate sections, but allows the dermatohistopathologist to create a list of differential diagnoses and appropriate comments (Stidworthy and Priestnall, 2011).
Histopathology in non-EL consists of diffuse dermal and SC infiltrates of malignant lymphocytes that are often CD4+ or CD8+ T-cell phenotype. Rarely, they are B-cell lymphoma (Day, 1995). The characteristic lesion in all forms of enteropathy-associated T-cell lymphoma is the tropism of neoplastic cells for the epidermis and adnexal structures (hair follicles, apocrine sweat and sebaceous glands). Mycosis fungoides histopathology showed 100% epitheliotropism of variable sized lymphocytes and sweat glands were infiltrated in 70% of cases (Fontaine et al, 2010).
Pautrier microabscesses, which are focal accumulations of lymphocytes, are regarded as common (Miller et al, 2013); however, they were found in only 23.3% of cases (Fontaine et al, 2010). SS includes presence of small lymphocytes with hyperconvoluted nuclei (Sézary cells), both within the epidermis and peripheral blood (Miller et al, 2013). Pagetoid reticulosis is characterised by almost exclusive infiltration of the epidermis by CD3+ and CD8+ T-cells (Miller et al, 2013).
In feline patients, these are also usually T-cell origin neoplasms. In Fontaine et al (2011), the characteristic lesions of feline EL were found to be similar to those reported in the dog, but involvement of the adnexal glands was not observed in this series (n=5). The neoplastic T-cells were generally small to medium in size.
Ideally, before commencing treatment, staging – including thoracic radiography, abdominal ultrasound, routine haematology, biochemistry and urinalysis – should be performed. This will allow identification of any abnormalities, including metastasis and other diseases, that may result in contraindication for certain therapeutic options.
The prognosis for dogs with nonepitheliotropic and epitheliotropic cutaneous lymphoma is extremely guarded to poor.
In general, most patients diagnosed with EL are euthanised because of the severity of their skin condition and poor quality of life. It is rare for patients with cutaneous EL to die naturally, unless the disease has progressed to generalised systemic lymphoma, development of SS or secondary septicaemia. The reported survival time for dogs suffering from EL ranges from months to two years, while it is suspected multiple subtypes of EL exist; therefore, explaining a long survival in some of our patients. This is yet to be officially documented.
It is often worth discussing case management with an oncologist or dermatologist before commencing treatment, as the treatment protocols can be complicated by secondary infections or systemic disease.
The mainstay of treatment for EL in veterinary patients is systemic chemotherapy with the oral alkylating agent lomustine. This can be administered at dose of 50mg/m2 to 70mg/m2 at three-weekly intervals until relapse, with a reported overall response rate of 78% to 83% (Williams et al, 2006; Risbon et al, 2006). Success is measured as complete response, partial response or stable disease.
Side effects of lomustine include myelosuppression (nadir 10 to 14 days post-treatment), gastrointestinal side effects and hepatotoxicity, which require close monitoring after every treatment and may result in cessation of chemotherapy.
On the whole, most cases of non-EL are part of a wider systemic disease process and, therefore, are best treated with chemotherapy, with the protocol often dependent on the localisation and immunophenotype.
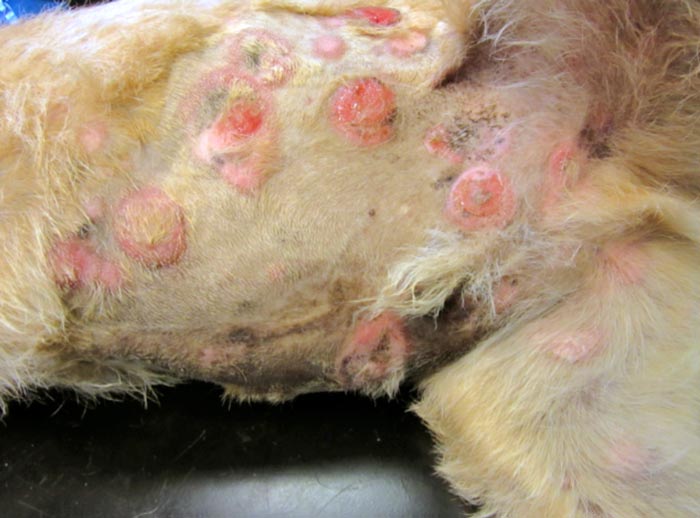
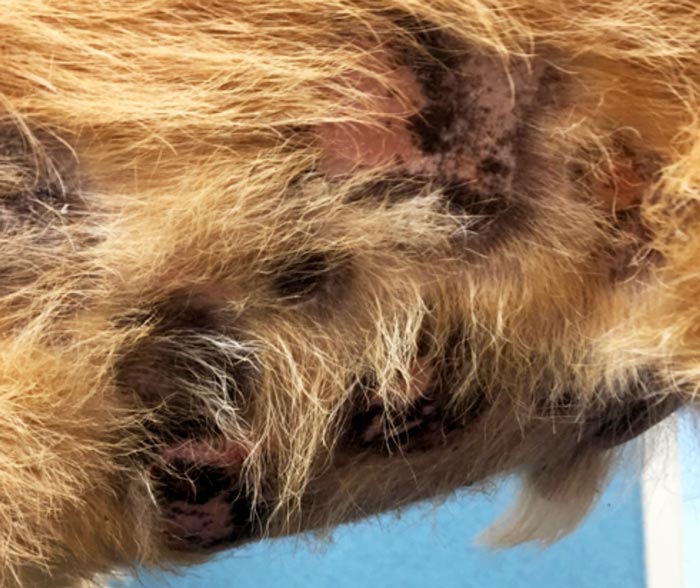
A study by Holtermann et al (2015) evaluated the efficacy and side effects of masitinib in the treatment EL. Of the 10 dogs in the trial, 2 underwent a complete remission and 5 underwent a partial remission. Masitinib is a tyrosine kinase inhibitor targeting kit receptor and platelet-derived growth factor receptor. Masitinib has also shown to have a mild anti-proliferative effect on canine lymphoid cells in vitro.
Synthetic retinoids (vitamin A analogues) can be used both topically (bexarotene) and systemically (isotretinoin and etretinate) for treatment of EL. They are often employed alongside systemic corticosteroid. A response rate to isotretinoin of 42% has been reported in a small number of dogs. Topical bexarotene cream is incredibly expensive and can cause cutaneous reaction (Miller et al, 2013). Furthermore, retinoids are teratogenic, so women of childbearing age should be careful when handling such medication.
Solitary lesions can be surgically excised, but, unfortunately, this is not commonly encountered, as the disease is often multifocal or generalised. Surgery can also be used as a palliative measure to remove lesions that are severely infected, pruritic or painful.
Radiation therapy can be used for both EL and non-EL especially for solitary or localised lesions and as palliative care for larger lesions (Miller et al, 2013).
The use of topical medications for the treatment of cutaneous lymphoma is commonplace in human oncology and is often practical for many owners with patients that have early clinical disease. This includes topical corticosteroids, hydrocortisone and triamcinolone (Miller et al, 2013).
Imiquimod, a topical immunomodulator, can be used for treatment of EL lesions if practical for the owner. A thin layer should be applied wearing gloves three times weekly. A pilot study performed in human oncology found histological clearance in three of the six patients treated with this regime (Deeths et al, 2005). In veterinary medicine it has, so far, been reported for use in two cases of melanocytomas (Coyner and Loeffler, 2011) and a retracted case report on preputial squamous cell carcinoma (Yaghoobi Yeganeh Manesh et al, 2014).
It has, however, been used for treatment in a wide variety of canine cutaneous diseases by veterinary dermatologists, although these are yet to be reported in the literature.
Treatment can also be complicated by secondary bacterial infections, which may also be multidrug resistant. This needs to be taken into account during treatment. Steroids are also used, either systemically or topically, in combination with other treatment protocols.
Finally, pain relief (such as paracetamol, gabapentin and tramadol) may also be required, as EL lesions can be painful and pruritic, especially in the more advanced stages.
Both EL and non-EL are uncommon cutaneous neoplasms that are often not diagnosed until clinical disease is advanced. Prompt recognition of clinical signs and biopsy with histopathology is required for early diagnosis.
The prognosis of our patients diagnosed with cutaneous lymphoma is dependent on the subtype and stage at which the disease is diagnosed. Multiple treatment options are available, which should be tailored to individual patients.