8 Jan 2018

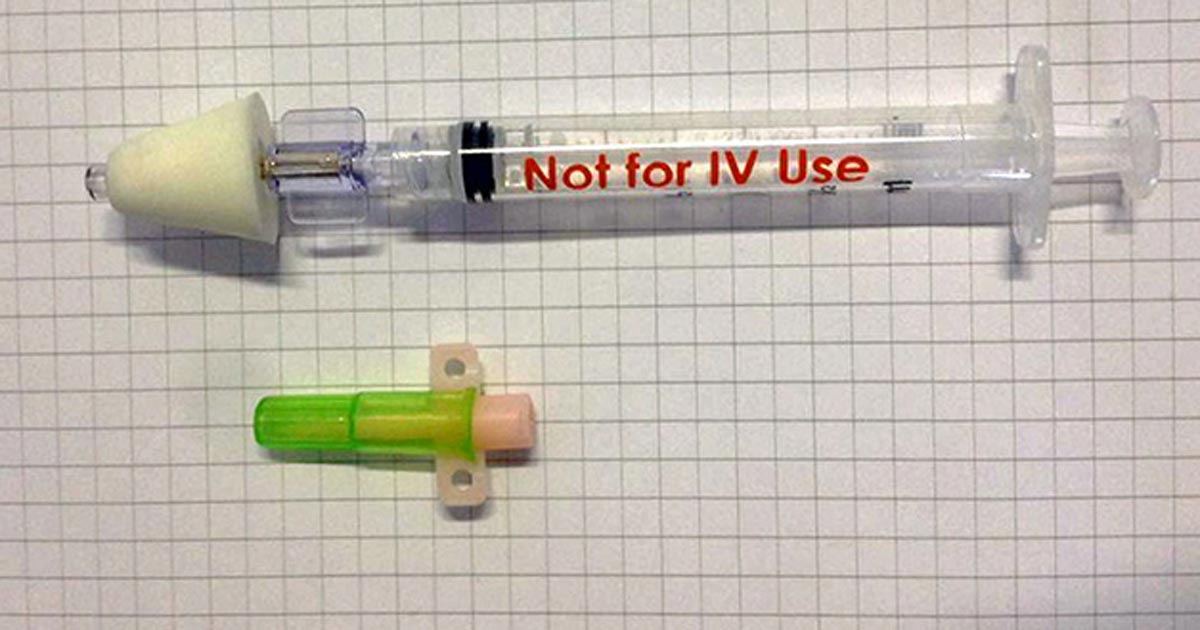
Figure 1. A mucosal administration device. The device consists of a syringe (1ml or 3ml) and, attached to the syringe, an atomizer. The latter turns the medication into a fine mist (30 microns to 100 microns).
Management of emergency epileptic seizures, such as status epilepticus, can be a common issue faced either by the owner at home or a vet at hospital. Initial and quick seizure cessation is considered important in these situations, as prolonged epileptic seizure activity can lead to permanent brain injury and systemic complications.
Benzodiazepines, including midazolam and diazepam, are commonly used as first-line medications in emergency epileptic seizures. When no IV access is available, rectal diazepam has been commonly used to cease epileptic activity. However, due to controversial results with regards to its effectiveness, an alternative route has been investigated in a clinical trial. In the latter, the clinical efficacy of intranasal midazolam as a first-line management option for canine status epilepticus was evaluated and compared to rectal diazepam for controlling status epilepticus before IV access is available.
The results showed intranasal midazolam was a quick, safe and effective first-line medication for controlling status epilepticus in dogs and appeared superior to rectal diazepam. Therefore, intranasal midazolam might be a valuable treatment option when no IV access is available and for treatment of status epilepticus in dogs at home. This finding was also supported by relevant human clinical trials.
Epileptic seizures can be considered emergency when multiple seizures occur close together over a period of 24 hours (cluster seizures), a manifestation of continuous epileptic seizures occurs lasting more than five minutes, or two or more discrete epileptic seizures with no incomplete recovery of consciousness between them occurs (status epilepticus)1.
Emergency epileptic seizures can occur either at home or in hospital, and should be urgently controlled2,3. Status epilepticus, in particular, can be a life-threatening condition in dogs and requires immediate management to avoid permanent damage to the brain4-8 or other severe systemic complications9. Therefore, effective initial (first-line) rapid management of emergency seizures, before administration of further antiepileptic drugs (AEDs), is vital.
Rectal (R), IM, buccal or sublingual administration may be useful – especially when IV access is not available15. IV administration of benzodiazepines provides good pharmacokinetic properties16 and is commonly used by vets as a first-line or second-line medication. However, when no IV access exists, R-DZP is a relatively easy and quick method by which owners at home or vets can attempt to terminate acute seizures.
Although R-DZP has been widely suggested as first-line medication, mainly to owners at home, controversial evidence exists with regards to its effectiveness12,17-20. Therefore, introducing evidence of another first-line treatment option that could potentially be more effective than R-DZP in ceasing emergency seizures was needed – especially at home.
Several human clinical trials have been performed to evaluate intranasal (IN)-MDZ in children and adults with emergency seizures, with or without comparison to R-DZP, and shown IN-MDZ is an effective and safe medication in the hospital and home settings15,21-33. In dogs, only pharmacokinetic studies have been performed and showed DZP16,34, MDZ35,36, triazolam and flurazepam36 were efficiently absorbed, and rapidly reached maximum serum concentrations following IN administration.
A multicentre, open-labelled, randomised, parallel-group clinical trial was performed to evaluate the clinical efficacy of IN-MDZ as a first-line management option for status epilepticus and compare IN-MDZ to the widely used R-DZP for controlling emergency seizures when no IV access exists.
The study was performed within a hospital environment, with the results anticipated to form the basis for using IN-MDZ for the treatment of emergency seizures at home. A nasal mucosal atomisation device (MAD; Figure 1), which converted the liquid drug into a fine mist, was used to effectively deliver the MDZ into the nasal cavity. The results of the clinical trial showed IN administration of MDZ is likely to be an effective, safe and easy method for short-term management of emergency seizures in dogs.
In this study, IN-MDZ was significantly more effective than R-DZP for terminating status epilepticus in hospital settings. It is likely this finding could be extrapolated to at-home treatment of emergency seizures before hospital admittance and, as such, IN-MDZ might be a more appropriate and effective pre-hospital seizure management method than R-DZP.
The anatomic structures of the nasal cavity, as well as the physiological and pharmacokinetic mechanisms of the IN administration of drugs, might provide an explanation for the effectiveness of IN-MDZ.
The nasal (sub)mucosa provides a large, highly vascular absorptive surface adjacent to the brain and offers a direct pathway for drug absorption into the bloodstream, avoiding the first-pass hepatic metabolism38-40. This is advantageous for benzodiazepines that need to rapidly accumulate in the brain during emergency management of seizures, but have a relatively short latency of action and undergo extensive hepatic metabolism.
MDZ is absorbed by the (sub)mucosal nasal vessels and enters the brain by crossing the blood-brain barrier (BBB), with a rapid clinical effect41. In addition, based on laboratory animal and human studies, some drugs, including benzodiazepines, might be able to reach the brain directly via the olfactory and trigeminal neural pathways39,40,42-46. The latter might be quite advantageous for refractory cases, where inadequate penetration of AEDs occurs across the BBB due to overexpression of drug vascular transporters, such as P-glycoprotein47.
These pharmacological advantages of IN administration of MDZ allow the drug to reach the systemic circulation more rapidly and may make it more effective in controlling emergency seizures with a lower “seizure cessation” time compared to the rectally administered drugs.
A mucosal atomization device was chosen in this study over a plain syringe for the IN drug administration because atomization of a liquid medication has been suggested to provide the benefit of increased and more rapid absorption37,48,49. The absorption rate and plasma concentration of drugs delivered by atomization has been suggested to be comparable to IV administration in some studies43,50,51.
In humans, this delivery method also results in a broader distribution of the medication across the nasal mucosa, an increased bioavailability and improved time to onset and efficacy of the drug with regards to seizure cessation15.
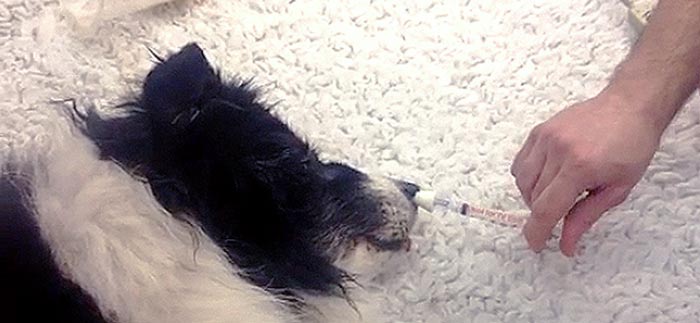
Finally, because the medication is atomized as a mist, it is less likely to overflow out of the nasal cavity compared to administration as a liquid form43,50,51. Indeed, in the current study, the MAD provided a simple and quick method for administering the medication. The soft edge of the device, which is in direct contact with the nostrils and the entrance of the nasal cavity (Figure 2), prevents expulsion of the drug and injuries to the soft tissues that could occur with the hard tip of a plain syringe in seizuring animals experiencing severe motor activity.
Based on the evidence, IN-MDZ might be an effective, quick and safe first-line medication for controlling canine status epilepticus and appears superior to R-DZP. The results support the use of IN-MDZ for treating emergency seizures – in particular, idiopathic epilepsy cases – prior to IV access. These findings could be extrapolated to treatment of emergency seizures at home prior to hospital admission.