3 Jul 2023

Image © chendongshan / Adobe Stock
The prevalence of chronic kidney disease (CKD) has been reported to be 13% in cats younger than four years, 2% in cats aged four to 10 years, 31% in cats aged 10 to 15 years and 32% in cats 15 years or older1. However, International Renal Interest Society (IRIS) stage 1 is often overlooked because the serum creatinine concentrations are within the normal reference interval for most laboratories.
CKD stage 1 can be identified by finding proteinuria, normoglycaemic glucosuria, isosthenuria (1.008 to 1.012), or poorly concentrated urine (1.013 to 1.035) and abnormal kidneys on palpation or on imaging. Using these criteria, more than 70% of cats between 15 and 20 years old, and about 35% to 40% of younger cats were found to have IRIS stage 1 or stage 2 CKD2.
In IRIS stage 1, the symmetric dimethylarginine values may be normal or elevated, and serum creatinine and urea values may have been increasing over time, though still within the reference range3.
Dietary therapy is the cornerstone for management of CKD in cats. Therapeutic feline renal diets improve survival time, reduce uraemic crisis episodes, improve serum urea nitrogen and phosphorus concentrations, and decrease fibroblast growth factor 23 (FGF-23), a hormone associated with poorer survival time.
When food intake is adequate, it may be possible for cats to maintain bodyweight and body condition score (BCS) for up to two years. Nutritional management with a renal diet is recommended in cats with IRIS stage 2 and higher; feeding renal diets in IRIS stage 1 is more controversial3.

Nutritional assessment as per WSAVA Global Nutrition Committee guidelines and toolkit should be performed at every visit4.
Bodyweight, a BCS and a muscle condition score should be performed at every consultation. Weight loss can precede the diagnosis of CKD; cats with a bodyweight less than 4.2kg at diagnosis reportedly have a shorter survival time than heavier cats5.
Muscle loss can be due to age-related sarcopenia in older cats, cachexia from disease-related inflammatory cytokines, and poor intake of energy, protein or amino acids6. Decreased muscle mass can decrease serum creatinine that can appear to be “improving” in a cat that is not clinically better.
Unrestricted access to water is essential to compensate for solute diuresis and urinary water loss. Older cats may not sense thirst well and may need encouragement to imbibe enough fluid to prevent dehydration.
Cats should have water bowls located in easy to access areas and possibly elevated. The bowls should be in several locations, and away from food bowls and litter boxes. Some cats like running water or water fountains.
Feeding canned food, possibly with added water, results in more fluid intake than a dry diet plus water. Using nutrient-enriched water can promote hydration in cats from nutritional osmolytes, which helps in the absorption of water at the cellular level.
Renal diets have high energy density – that is, they are usually low fibre and high fat. Fat is a palatable and high calorie nutrient, which helps increase calorie intake. While cats fed commercial renal diets have significantly better survival than those on maintenance diets, some will refuse to eat a renal diet. Early use of renal diets (for example, during IRIS stage 1) and a slow transition can improve compliance. In one study, the transition period ran for six weeks or longer7.
In this study, 94% of cats were successfully transitioned to a therapeutic diet, defined as 80% of calorie intake from the renal diet.
If the cat refuses to eat the therapeutic diet, a homemade diet formulated for kidney disease by a nutritionist may be tried. Nearly all homemade diets in books and online are not complete and balanced for cats with CKD8.
While some cats with CKD may be overweight, most of them are at risk for disease-related weight loss.
While dietary protein restriction has previously been advocated for CKD management, the optimal amount of protein for cats with CKD is not known.
Protein-restricted diets are usually phosphorus restricted, and the effects of the two nutrients are difficult to separate. Lower-protein diets were once thought to slow the rate of CKD progression, although this concept is unproven and again, the effects may be due to lower phosphorus. Higher protein diets do not appear to increase the risk of developing CKD.
The National Research Council adult feline minimum protein requirement9 is 40g/1,000kcal; the European Pet Food Industry (FEDIAF) minimum protein requirements for adult cats are 62.5g/1,000kcal metabolisable energy (ME) and 83.3g/1,000kcal ME using maintenance energy requirements (MER) of 100kcal/kgBW0.67, and 75kcal/kgBW0.67, respectively10.
Diets formulated for renal disease are above the FEDIAF lower limits for protein on a dry matter basis (DMB). They are also usually just above the FEDIAF protein requirement of 62.5g protein/1,000kcal ME content for cats with a higher energy requirement (100kcal ME/kg0.67). For cats with lower energy intake (75kcal ME/kg0.67), some diets may fall below the 83.3g protein/1,000kcal FEDIAF protein minimum.
Most renal diets provide at least the minimum amount of protein or more, and should provide adequate amino acids. Protein quality and digestibility are also important; lower protein amounts may be sufficient with high protein digestibility and essential amino acid, plus enough of the non-essential amino acids.
Protein digestion is decreased in 20% of cats of 14 years of age, and restricting protein may contribute to loss of lean body mass11, which can predict survival time. In healthy cats, protein of 5.2g/kg bodyweight (7.8 g/kg BW0.75) is needed to maintain lean body mass12.
In 28 cats with renal disease, a higher protein diet (9g/kg/day versus 5.2g/kg/day) fed for 12 months was not associated with increased severity of glomerular or non-glomerular renal lesions, increased proteinuria, or decreased glomerular filtration rate13.
A renal diet with added essential amino acids and carnitine, as well as increased calories, helped maintain bodyweight and lean body mass (LBM) in cats with IRIS stage 1 and stage 2 CKD14.
Renal diets restricted in protein and phosphorus do increase survival time in cats with CKD, although this may be due more to the phosphorus restriction.
Separating the effects of dietary protein and phosphorus is difficult, and many higher protein diets are high in phosphorus.
Controlled restriction of non-essential protein will reduce the accumulation of nitrogenous waste products contributing to uraemic syndrome, and may also decrease acidosis.
Cats in IRIS stage 2 or stage 3 fed a renal diet had decreased serum urea nitrogen and increased blood bicarbonate, although no difference was recorded in serum creatinine, potassium, calcium, parathyroid hormone (PTH) concentration or urine protein: creatinine (UPC) ratio.
Cats on the renal diet had fewer uraemic episodes and renal disease-associated deaths7. Protein recommendations in cats with IRIS stages 2 to 4 CKD vary from 28% to 35% DMB, and 20% to 40% of protein calories15.
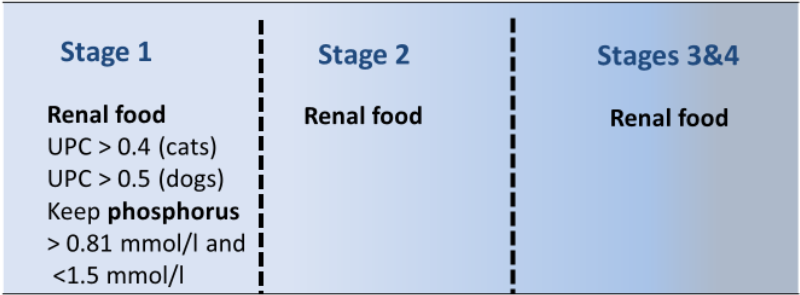
Introducing a diet change early does improve diet acceptance. As noted, more than 90% of cats with CKD accepted renal diets using a very gradual transition16. In most cases with significant proteinuria – that is, a UPC ratio of more than 0.4 – or serum phosphorus above the reference range, feeding a renal diet is indicated17.
For cats without proteinuria or hyperphosphataemia, the indication for feeding restricted protein for early renal disease is less clear.
Feeding a renal diet to cats with CKD showed renal parameters (serum creatinine and blood urea nitrogen; BUN) of IRIS stage 1 cats were unchanged between baseline and after 12 months14.
This study was not controlled; however, renal disease usually progresses, so the lack of progression is promising.
Renal diets for earlier CKD stages contain moderate protein and restricted phosphorus concentrations. In some cases, a senior diet that meets the nutrient requirements for early renal disease could be considered, although the early stage renal diets will likely have other benefits, such as improving acid-base balance, increased B vitamins, omega-3 fatty acids, carnitine, and antioxidants, and would usually be the preferred choice. Further, not all senior diets are appropriate for early CKD.
Renal hyperparathyroidism (RHPTH) is implicated in disease progression, as well as contributing to the uraemia. The main cause of RHPTH is absorption of dietary phosphorus exceeding the excretion ability of the diseased kidneys.
Phosphorus retention initially increases FGF-23 from fibroblasts and later increases synthesis of PTH. FGF-23 decreases phosphorus reabsorption in the proximal renal tubule, decreases intestinal phosphate absorption via decreased conversion of calcidiol to active vitamin D – calcitriol – and increases synthesis of PTH.
PTH also decreases renal phosphorus absorption, but increases the synthesis of calcitriol from calcidiol. Initially, these effects maintain serum phosphorus in the reference range at the expense of increased serum PTH and FGF-23.
Some IRIS stage 1 cats may have elevated serum FGF-23 prior to azotaemia and increased PTH prior to hyperphosphataemia. All IRIS stage 4 patients have RHPTH.
PTH stimulates osteoclast activity to release calcium to help correct hypocalcaemia, causing demineralisation of bone.
Increased serum phosphorus and calcium concentrations can lead to soft tissue mineralisation. The treatment goals are to restrict phosphate intake, prevent or decrease bone loss, and prevent or decrease soft tissue mineralisation. Restricting dietary phosphorus significantly increases survival time in CKD16,18-19.
As well as effects on phosphorus and PTH, a phosphorus-restricted diet helps reduce plasma FGF-23 concentrations in hyperphosphataemic and normophosphataemic cats with stable, azotaemic CKD20.
Recommended dietary phosphorus concentrations for cats are 0.3% to 0.6% DMB, or 1g/1,000kcal. Recent research indicates the phosphorus form – that is, what it is complexed with, such as sodium dihydrogen phosphate (for example, vs bone meal) – affects the kidneys.
The more soluble or “inorganic” forms have negative effects on renal, as well as cardiac and skeletal health via their effects on calcium and phosphorus homeostasis.
The intake of a diet with excessive highly available phosphorus has an adverse effects on parameters of kidney function, even in healthy cats21. The calcium to phosphorus (Ca:P) ratio is also important, with low ratios more damaging and a recommended Ca:P of more than 1.0.
Cats with serum phosphate within the IRIS target range may be at increased risk of hypercalcaemia when fed very low phosphorus diets.
If total serum calcium exceeds 3mmol/L or ionised calcium is above the reference range, more moderate phosphate restriction should be introduced; for example, an early renal diet, a senior diet, or a renal diet mixed (such as 50:50) with a maintenance diet.
While sometimes necessary, adding phosphate binders to a maintenance or senior diet is not ideal, as control of serum phosphorus is seldom as good as when feeding a renal diet.
Treatment with an active form of vitamin D (calcitriol or alphacalcidol) has controversially been recommended to directly inhibit PTH secretion. This should be used only after the serum phosphorus has been controlled and may be indicated more in later stages.
These drugs have a narrow therapeutic index due to their tendency to cause hypercalcaemia, and careful monitoring is needed.
Potassium varies among renal diets (from about 1.5g/1,000kcal to 3.5g/1,000kcal). The diet choice depends on the patient, as it may be hypokalaemic or hyperkalaemic.
When hyperkalaemia occurs while the cat is on a renal diet, appropriately formulated, potassium-reduced, homemade diets can be an effective alternative. Hypokalaemia occurs in 20% to 30% of cats with CKD. Hypokalaemia has been associated with muscle weakness (especially cervical ventroflexion), and morphological renal abnormalities, which can worsen CKD.
Metabolic acidosis is common with CKD, as the kidneys may be less able to reabsorb bicarbonate. Acidosis can cause decreased appetite, vomiting, lethargy, weakness, and increased protein catabolism.
IRIS guidelines are for blood bicarbonate to be maintained between 16mmol/L and 25mmol/L in a stable and hydrated patient3. Most renal diets are alkalinising.
Increased dietary sodium was associated with increased azotaemia in one feline study22, and is thought to contribute to signs of uraemia in people. This concept is still controversial: a two-year study in older healthy cats found no association between salt consumption and glomerular filtration rate23.
Recommended sodium levels in cases of feline CKD are thought to be between 0.2 DMB to 0.35 DMB15. Blood pressure in cats does not appear to be affected by sodium unless it is at excessively high levels. Highly restricted sodium intake activates the renin-angiotensin-aldosterone system, potentially worsening CKD progression and increasing potassium loss.
As B vitamins are water soluble, increased urine output can increase loss.
Cats have an increased B vitamin requirement compared to dogs, and supplementation, present in most renal diets, is recommended.
Commercial renal diets are often high fat, which increases caloric density, palatability and, therefore, can help maintain bodyweight.
The omega-3 fatty acids, eicosapentaenoic and docosahexaenoic acid, modify the inflammatory response and are likely useful in CKD.
Omega-3 fatty acid supplementation may reduce glomerular hypertension, proteinuria and limit production of inflammatory mediators (for example, prostaglandin E2 and thromboxane A1).
A study of seven feline renal diets showed the most effective diet for survival time had a high content of eicosapentaenoic acid24, and many renal diets now include increased omega-3 fatty acids.
Palatability of omega-3 supplements seems to vary among cats and it is often better as an integral part of the diet.
Renal oxidant stress likely plays a role in progression of CKD and renal fibrosis.
Chronic hypoxia and renal ischaemia, as a result of either reduction in renal perfusion and/or anaemia, may exacerbate renal injury and create a pro-inflammatory environment. Added antioxidant vitamins E and C, and beta-carotene, reduced DNA damage in cats with renal insufficiency25.
Modulating the intestinal microbiota using dietary fibres (such as prebiotics) and/or probiotics may reduce some uraemic toxins (such as serum BUN, creatinine or p-Cresyl sulphate), as probiotic bacteria may catabolise urea and uraemic toxins, trapping them in the bowel lumen to be excreted in faeces.
Randomised placebo controlled studies are warranted to determine if these treatments can affect quality of life and survival time for cats with CKD26.
The importance of monitoring cannot be overemphasised.
Response to any therapeutic intervention must be carefully assessed, both in terms of clinical improvement and impact on disease progression.
In addition to standard CKD, monitoring and regular nutritional evaluations are vital.
Owner education and communication improves understanding and compliance, with the ultimate goals of improving quality of life and longevity for their cat.