17 Mar 2020

Figure 1. Crenosoma first stage larvae.
Increased pet travel, importation of pets and the expansion of global parasite distributions is increasing the risk of pets infected with exotic parasites entering the UK. Rapid recognition of clinical signs, effective use of preventive treatments and screening tests in imported dogs are vital components of improving prognostic outcomes in these animals, as well as limiting parasite spread and zoonotic risk.
This article considers a systematic approach to exotic parasites and the imported dog, using European Scientific Counsel Companion Animal Parasites UK and Ireland’s “four pillars” in clinical practice.
Since the Pet Travel Scheme was relaxed in 2012, pet travel has increased year-on-year alongside growing numbers of legally and illegally imported pets entering the UK from abroad.
Part of this trend has been driven by pets being rescued from abroad and then being rehomed in the UK. This, in combination with expanding global parasite distributions, increases the risk of exotic parasites being introduced.
Novel parasites may establish in a number of different ways.
Introduction of parasites into existing vector populations; “vectors waiting for a disease”. Dermacentor reticulatus and Ixodes species ticks are already endemic in the UK, and are vectors of Babesia canis and tick-borne encephalitis, respectively. Mosquitoes capable of transmitting the skin worm Dirofilaria repens and heartworm Dirofilaria immitis are also present in the UK. These existing vector populations may then become infected through feeding on infected pets that have travelled abroad or, in the case of tick-borne diseases, through infected ticks being introduced on imported animals. This is not just a theoretical risk, with endemic foci of B canis (Phipps et al, 2016) and tick-borne encephalitis already having established in existing UK tick populations.
Introduction of parasites into areas where vectors have spread. New vectors such as Phortica species fruit flies have arrived in the UK that could harbour novel parasites, such as the eye worm Thelazia callipaeda. This parasite is arriving in the UK though travelled and imported pets (Graham-Brown et al, 2017), presenting the opportunity for UK fruit flies to be infected.
Introduction of vectors and parasites together. Although Rhipicephalus sanguineus ticks are not endemic in the UK, they are frequently seen on travelled dogs. These ticks may be infected with exotic tick-borne pathogens such as Ehrlichia canis, and zoonotic rickettsia potentially exposing pets and people through household infestation (Hansford et al, 2015).
Introduction of vector-borne parasites that are then transmitted in the absence of the vector. Leishmania infantum has established in countries such as Canada, with no sand fly vector to transmit through other forms of transmission such as venereal, congenital, blood transfusion and possibly dog bites. Cases of transmission have also occurred in the absence of the sand fly vector (McKenna 2019; Wright and Baker, 2019).
The rapid identification of parasitic infection and disease in imported rescue dogs forms an essential part of preventing new parasites and vectors establishing in the UK through these routes.
Due to the ongoing risk imported parasites represent to individual pets, owners, the wider public and UK biosecurity, European Scientific Counsel Companion Animal Parasites UK and Ireland recommends a systematic approach to imported dogs in practice, consisting of four key steps (the “four pillars”).

Identification of ticks allows the introduction and distribution of exotic tick species to be monitored in the UK, but also indicates which tick-borne diseases the imported pet may have been exposed to.
Ticks should be removed with a tick removal device or fine pointed tweezers. If tweezers are used, the tick should be removed with a smooth upward pulling action. If a tick hook is used, then a simple “twist and pull” action is employed.
Compressing or crushing ticks in situ with blunt tweezers or fingers will stress the tick, leading to regurgitation and emptying of the salivary glands, potentially leading to increased disease transmission. Traditional techniques to loosen the tick, such as the application of petroleum jellies and burning, will also increase this likelihood and are contraindicated.
Adult ticks can be examined by the naked eye to identify gross features. Anatomical features can be examined more closely under a dissecting/stereo microscope – ×10 or ×40 magnification using “top” incident light. The tick may be mounted on a small circle of Blu-Tack for easy manipulation under the microscope.
Larval ticks and nymphs are smaller and will require microscopic examination for identification. They can be placed in a drop of water or liquid paraffin on a slide, under a coverslip. Features can then be examined under a ×4 or ×10 objective lens.
The first step in identification is to confirm the ectoparasite is a tick and not a mite. Adults and nymphs are usually large enough to distinguish them on size. Larval ticks are similar in size, but only have six legs and have mouthparts unique to ticks, known as a hypostome (Figure 1).
Ixodes species ticks can be differentiated from other genus of ticks by the anal groove, which, in Ixodes ticks, is anterior to the anus (Figure 1). They also lack any decoration possessed by other ticks, such as festoons, eyes or scutal decoration. Dermacentor and Rhipicephalus species have a groove posterior, and no groove anterior, to the anus. In addition, these ticks and other exotic genera, such as Hyalomma, have decorations that can be used for identification, such as festoons, eyes and decoration on the scutum. Festoons are crimping around the edge of the abdomen, giving a Cornish pasty-type appearance (Figure 2). Eyes are spots present symmetrically either side of the scutum.
Ticks can be identified to genus using these features, using the University of Bristol tick identification site. The Public Health England Tick Surveillance Scheme is also happy to receive ticks for identification. Ticks should be placed in a container with a secure lid and sent in an envelope marked “biological sample”. Samples should ideally be unfed or only partially engorged as features on the scutum and festoons may be obscured in fed ticks.
More information can be found on the GOV.UK website. Using this service also allows the ticks to be included in real-time data, contributing to an overall picture of which species of ticks are being brought into the UK and by what means.

Treating dogs again with praziquantel within 30 days of return to the UK – and for ticks if treatment is not already in place – will ensure Echinococcus multilocularis is eliminated from imported pets, whatever the timing of their compulsory treatment, or if doubt exists about it having been administered.
E multilocularis-infected dogs in the UK represent a threat to both national biosecurity, and a significant zoonotic threat to individuals living with, and coming into contact with, the dog.
Tick treatment will increase the likelihood of attached ticks being killed if they are missed on examination. Ticks are easily missed in large or long-coated breeds, or those that are reluctant to be examined.
A thorough and comprehensive clinical exam of imported pets will identify adverse clinical signs, and these can then be compared to common exotic parasitic diseases and those in the countries the pet has visited.
Clinical signs typically associated with imported tick-borne disease include:
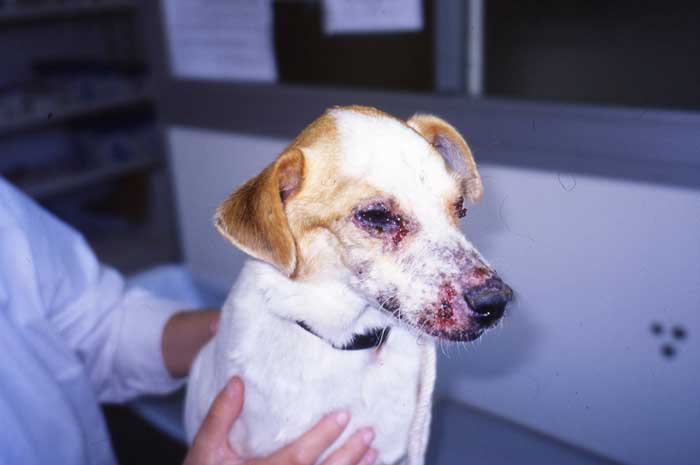
Anaemia and thrombocytopenia. Babesia species infection can lead to immune-mediated haemolytic anaemia in dogs, with subsequent regenerative anaemias developing. These most commonly are acute, and typically present pale mucous membranes, icterus, fever and hepato-splenomegaly. Associated depression and anorexia may be present, as well as dark brown urine associated with haemoglobinuria (Figure 3). Concurrent thrombocytopenia may be present with petechiation on the gums, spontaneous bleeding or bruising (Figure 4). Imported and travelled dogs with these signs should raise suspicion of Babesia species infection. Travel history will often be present for acute infections, but may have occurred months or years previously – as infection with Babesia species is often lifelong and relapses of clinical disease common. Anaplasma platys is a cause of cyclic thrombocytopenia in dogs, so this should be considered as differential diagnosis in travelled dogs suffering from recurrent bouts of thrombocytopenia. It is also a common sign in chronic ehrlichiosis.

Lymphadenopathy and pyrexia. Many clinical tick-borne infections transmitted by R sanguineus will present acutely with lymphadenopathy and pyrexia including E canis, Hepatazoon species, A platys and Babesia vogelli. Travelled and imported dogs presenting with these signs should be tested for these parasites, and checked for R sanguineus ticks as house infestation is a possibility. It is also important to recognise these acute signs of E canis infection, as without treatment it may progress to the chronic, often fatal form in dogs.
Neurological signs. Tick-borne encephalitis, and both acute and chronic ehrlichiosis, may present with signs associated with meningitis and meningoencephalitis. These include ataxia, seizures, paresis, hyperaesthesia, cranial nerve deficits and vestibular signs. These may present in dogs with a recent history of travel – or, in the case of chronic ehrlichiosis, months or years previously.
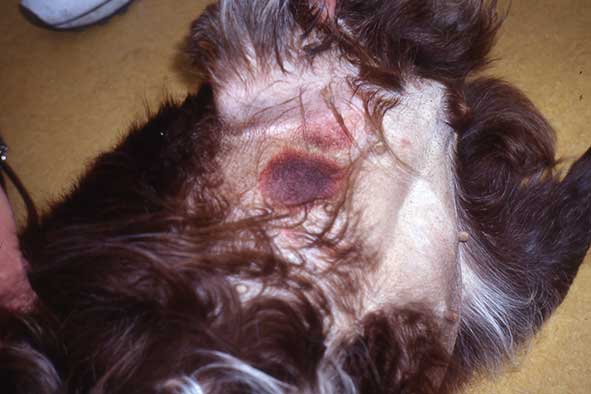
Leishmaniosis is chronic in nature, with a variety of presentations and periods of remission. Signs are due to immune complex deposition in various organs, and include lymphadenopathy, cutaneous signs (for example, generalised and focal alopecia, hyperkeratosis, dermal ulcers and periocular alopecia; (Figure 5), weight loss, splenomegaly and renal signs associated with glomerulonephritis. Less commonly polyarthritis, thrombocytopenia, ocular inclusion bodies, uveitis, and neurological signs associated with spinal and CNS granulomas may be present.
Leishmaniosis should be considered as a differential in imported dogs, as well as those with travel history. Signs may take months or years to develop, so foreign travel may not be recent. Infected pets may be subclinical, and mixed infections with tick-borne pathogens are common, so a pet positive for Leishmania infection may have other infections responsible for presenting clinical signs.
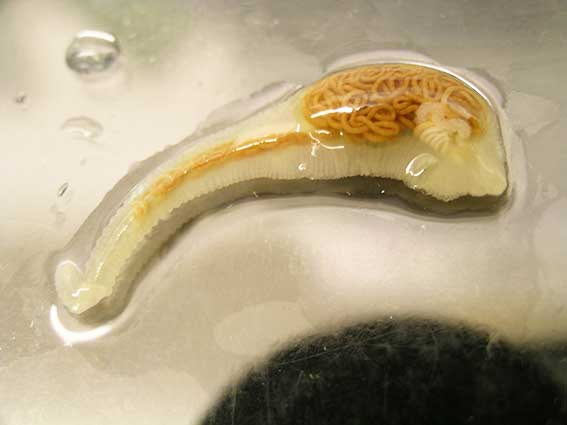
Although often subclinical, ocular thelaziosis can commonly cause conjunctivitis, keratitis, ephiphora, eyelid oedema, corneal ulceration and, in serious cases, blindness. Close examination of the conjunctiva will often reveal worms actively moving on the surface (Graham-Brown et al, 2017) and checking is vital in all imported cats and dogs to detect low-grade or subclinical infections to prevent vector exposure.
Coughing, tachypnoea, dyspnoea and exercise intolerance are the most common clinical signs seen in infected dogs. Acute clinical signs are associated with thromboembolism, subsequent pulmonary hypertension and caval syndrome. Worm death can also lead to thromboembolism and anaphylaxis. Typical resulting acute clinical signs include sudden death, anorexia, weakness, dyspnoea, vomiting and, rarely, respiratory signs linked to pleural effusion.
Cases of D repens infection are most commonly subclinical, but clinical signs associated with infection can occur. Dermatitis is the most common clinical presentation as multifocal nodules in the skin or papular dermatitis. These signs can reoccur seasonally for years after infection resulting in pruritus, erythema, papules and focal or multifocal alopecia. Less commonly, hyperkeratosis, crusting, distinct nodules, acanthosis and secondary pyoderma can occur.
Signs may also develop from migration of worms to other parts of the body including conjunctivitis, anorexia, vomiting, fever, lethargy and lymphadenopathy. These signs are partially immune mediated in nature, and partially due to disruption and irritation caused by physical movement of the worms.
Ocular migration of worms into the vitreous is uncommon, but does occur and, therefore, D repens should be considered as a differential if worms are visualised there and in dogs presenting with dermatitis that have travelled abroad.
A number of nasal pentastomid L serrata (tongue worm) cases have been seen in the UK over the past four years, from dogs imported from Romania and, more recently, Turkey (Mitchell et al, 2016; Figure 6). Infection is acquired from the consumption of raw meat and offal in endemic countries. L serrata is a zoonotic infection with humans acting as intermediate, as well as definitive, hosts and may, therefore, become infected through ingestion of eggs from nasal secretions and faeces.
Most cases of infection in dogs and cats are subclinical. However, large burdens can lead to rhinitis and nasopharyngitis with associated chronic sneezing and/or coughing, purulent nasal discharge and epistaxis. It is vital these signs are detected early in affected dogs to limit zoonotic exposure to owners and others in contact who may ingest infective eggs in nasal discharge or from faecal contamination.
Both L infantum, tick-borne diseases and heartworm can have long incubation periods and carrier states. Infection can also be lifelong and, in some cases, carry a poor prognosis.
Screening for these parasites in dogs imported from endemic countries will lead to early diagnosis, preparing the owner for what could be a lifetime of potential treatment, any associated zoonotic risk and limiting wider spread through effective tick control.
Light microscopy of blood smears is a useful test to perform in all imported dogs. Dogs infected with Hepatazoon canis typically have high numbers of gametocytes present in peripheral blood smears (Figure 7). Piroplasms of Babesia species in red blood cells, as well as morulae of E canis and A platys, may also be seen.
If samples are not going to be prepared immediately, or if samples are going to be sent to an external lab for blood smear preparation, blood samples can be stored in ethylenediamine tetra-acetic acid tubes. Alternatively, samples can be prepared and air dried without fixing and staining before sending to an external lab. Parasites may be seen in red or white blood cells or extracellularly.
Blood smear examination carries a high sensitivity and specificity for H canis detection and B canis in clinical cases. Screening for E canis and A platys, however, requires PCR or serology testing.
Serology is highly sensitive and specific for detecting exposure to the parasite. Quantitative serology is useful in the case of E canis infection, where a four-fold increase in test titres taken two weeks apart is indicative of active infection. Blood PCR is also a highly specific and sensitive test for both parasites.
Clinically affected dogs can be tested by quantitative serology, PCR or histology of affected tissues. In the sub-clinical carrier, quantitative serology over time is useful in monitoring for emerging active infection. Serology is less sensitive, however, in the first few months post-infection. Conjunctival swab PCR is a useful non-invasive test, with approximately 85% sensitivity.
Testing for uterine antigen secretions of female adult D immitis heartworms is a highly specific diagnostic test in infected dogs and highly sensitive in cases of large worm burdens.
A second test in newly imported animals six months after arrival is useful to rule out infection. The modified Knott’s test carried out be some external labs concentrates microfilariae in the blood and is a useful test in positive dogs to quantify the numbers of circulating microfilariae. Dogs with high circulating numbers are more likely to suffer anaphylaxis if treated with macrocyclic lactones. Microfilariae of D repens will also be detected by this method.
The growing trend of importation of rescue dogs from abroad is only likely to grow over the coming months and years.
While, as a profession, vets have a role to play in educating the public regarding the disease risks of importing dogs from abroad, veterinary professionals also have a vital role in assessing imported dogs for evidence of parasitic infection and putting effective preventive measures in place.
In doing so, individual pet and human health is improved, but also that of UK dogs and human population as a whole.