18 Mar 2025

Figure 1. The contributing factors that play a role in canine atopic dermatitis.
Canine atopic dermatitis (CAD) is a genetically predisposed, chronic, pruritic, inflammatory skin disease. CAD affects approximately 10% of patients1, so in a normal working week in companion animal practice, these patients will make up a significant proportion of the caseload.
Previously, CAD was defined by the International Committee on Allergic Diseases of Animals (ICADA) as “genetically predisposed inflammatory and pruritic allergic skin disease with characteristic clinical features associated most commonly with [immunoglobulin E] antibodies to environmental allergens”.
More recently, the ICADA has changed the definition to “predominantly T cell-driven inflammatory skin disease involving an interplay between skin barrier abnormalities, allergen sensitisation and microbial dysbiosis” to reflect the change in our understanding of the pathogenesis of this disease2.
CAD is a multifactorial disease (Figure 1) which involves aberrations in multiple areas including skin barrier, immune system, microbiome; approximately 80% of CAD patients have an allergic component to their skin disease.
Therefore, we should look to manage as many of these contributing factors where possible.
Due to the impact on both pet and owner quality of life with management of this condition, it is imperative that we do our utmost with the options available to us3. Investigation and diagnosis of CAD is outside the scope of this article, but numerous Vet Times articles available, as well as an open access research paper4.
It is always worth noting that CAD is not a static disease, and peaks and troughs will be encountered that will require management, and some of these will have seasonal factors.
Treatment can be split into reactive and proactive therapy, reflecting the current trend in human dermatology5.
Reactive treatment is associated with getting inflammation and pruritus quickly under control by using broad-based treatment. This can be likened to being in choppy waters and bringing a boat into a calm harbour.
Proactive treatment is associated with maintenance of disease and preventing recurrence, and this can include both broad-based and narrow-based therapeutics. Again, using water as an analogy, it is akin to sailing on calmer waters.
Treatments used at these separate stages may be the same, but at differing doses such as glucocorticoids (GC), or they involve different agents such as GCs to induce remission and long-term oclacitinib (OCA) for proactive treatment.
In this article, we will look at the therapeutics available, their mode of action, and some of the pitfalls we can encounter when using them.
Discussing these elements with owners and allowing them to feel part of the decision-making process can empower them with their choice, and can help with adherence to the treatment plan.
Reactive therapeutics are broad-based treatments that aim to quickly get inflammation and pruritus under control. Both systemic and topical GCs, as well as OCA, can be used to induce remission depending on the severity of the patient’s lesions.
In those patients with mild to moderate erythema, inflammation and pruritus, either GCs or OCA can be utilised, whereas in those with moderate to severe clinical signs, GCs are likely to be more appropriate unless a contraindication to their use exists.
GC treatment given via systemic and/or topical routes remains the foundation of management of CAD for many patients.
GCs are fast acting and inexpensive, and use results in a rapid reduction in production of inflammatory cells and mediators; therefore, effectively controlling inflammation and pruritus6. The effect of GCs is associated with the presence of glucocorticoid receptors in almost every cell within the body. This results in reduction in transcription of mRNA, subsequent translation in the Golgi apparatus, and reduction in cell function including cytokine production.
This is the equivalent of casting a very wide, tightly woven fishing net and removing as many of the cells and the mediators that are contributing to inflammation and itch as possible.
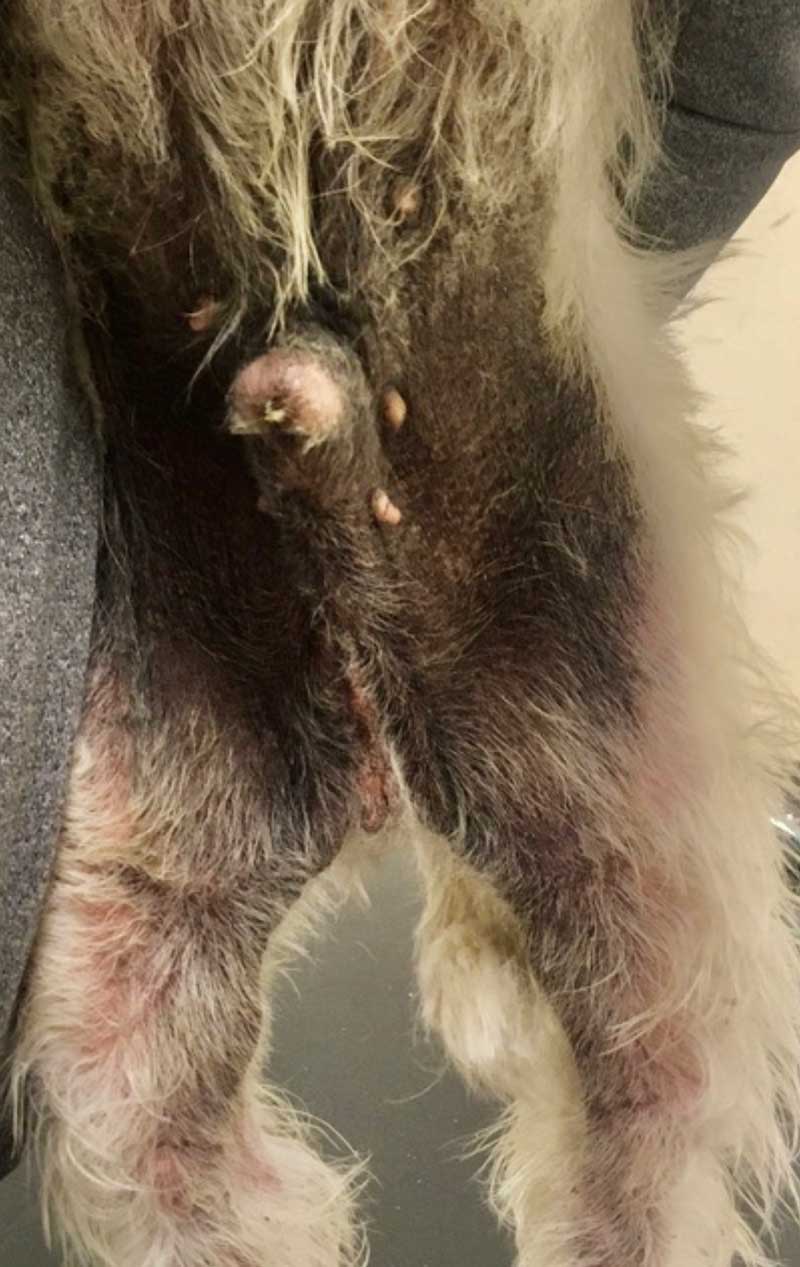
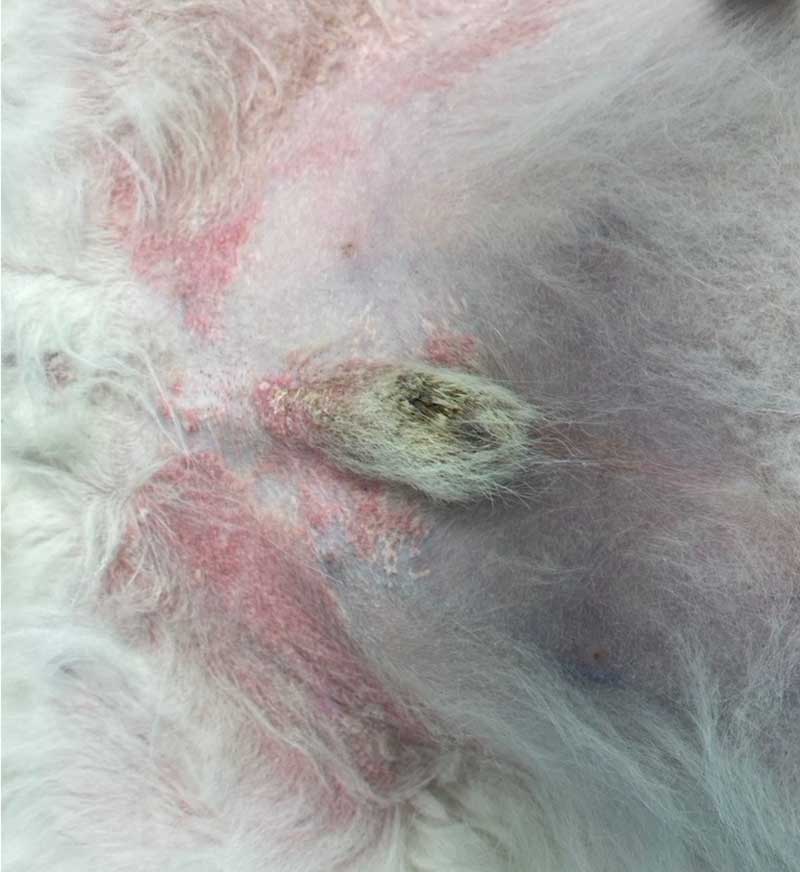
However, adverse effects associated with GC use can include polyuria/polydipsia, polyphagia, behaviour change, muscle wastage, weight gain and skin atrophy among others, and this can be undesirable. Development of calcinosis cutis (Figure 3) can occur with both systemic and topical GCs, and is not dose or duration dependent in the author’s experience. If long-term GC treatment is to be considered, then tapering to determine the most effective dose should be performed7.
Adjuncts to GC therapy can be considered to allow a dose reduction and this includes the use of antihistamines (off licence in the UK), and/or essential fatty acids can be utilised to allow dose reduction8,9.
OCA is a Janus kinase (JAK) inhibitor, which predominantly targets JAK1, utilising the Janus kinase and signal transducer and activation of transcription (JAK-STAT) pathway. It is licensed for treatment of clinical signs of CAD, as well as pruritus associated with allergic dermatitis in dogs older than 12 months.
OCA acts to reduce signalling of key cytokines utilising this pathway, including IL-2, IL-4, IL-13 and IL-31. Going back to the fishing net analogy, this is similar to using one with different shaped holes, which means only a proportion of fish are caught.
In this case, cells that utilise JAK-STAT pathway will be affected.
OCA is as fast acting as GC, and an effective therapeutic with a good safety profile that has been utilised for more than 10 years. OCA can be used in both reactive and proactive treatment. Given its mechanism of action and short half-life (four to six hours), tapering of the dose interval is rarely possible, but it can be possible to reduce the daily dose in some patients.
Common side effects associated with OCA include vomiting, diarrhoea, otitis, lethargy, weight gain and pyoderma10. Its use in patients with demodicosis, severe infections and a history of neoplasia is strongly discouraged. Patients should be monitored for development of cutaneous masses, and fine-needle aspirate biopsy should be considered in pets that develop a mass.
An abstract published in 2017 by High and colleagues reported an increase in histiocytomas in patients on long-term OCA (2.6%) compared to those on CSA (0.6%)11. Lancelotti and colleagues looked at the medical records of 660 dogs, with 339 dogs on OCA for at least six months with a minimum of two-year follow ups with age and breed matched controls (321 dogs). They did not find that OCA therapy posed an increased risk to neoplasia development12.
In patients on long-term treatment, monitoring of biochemistry, haematology and urinalysis is advised due to rare occasions of haematological and biochemistry changes, proteinuria and urinary tract infections10, 13.
Proactive agents are those that are employed to help prevent recurrence of disease. These can be narrow spectrum or targeted therapeutics, such as lokivetmab (LKV) or allergen-specific immunotherapy, or they can be agents with a wider range of activity, such as CSA or GC.
Both GC and OCA can be used in proactive treatment of CAD, with the former titrated to lowest effective dose. These two medications are discussed in the section on reactive treatment.
CSA is a systemic calcineurin inhibitor licensed for use in CAD patients and has been utilised in veterinary medicine for more than 20 years. It acts to block pro-inflammatory cytokines and pathways by binding to cyclophilin, preventing dephosphorylation of nuclear factor of activated T cells (NFAT); therefore, preventing NFAT translocation to the nucleus and subsequent gene transcription.
The impact of this is primarily a reduction in cytokines such as IL2, IL4 and interferon gamma (IFN-y); therefore, reducing the number and activity of antigen-presenting cells (APC) and mast cells and reduction in immunoglobulin E producing B cells, among others14.
Due to its efficacy on a larger number of cells, due to presence of NFAT within them, CSA is considered more anti-inflammatory than OCA, but not as anti-inflammatory as GCs.
As CSA takes several weeks for efficacy, it cannot be utilised in reactive therapy when a quick acting therapeutic is needed. During the time period before efficacy, CSA can be combined with oral GCs or OCA for a short period of time – usually as a two-to-three week tapering course15, 16.
Short-time side effects associated with CSA use can include vomiting and diarrhoea, which is usually transient and does not require treatment. This can be reduced by giving CSA with food or freezing the capsules beforehand; this does not impact the clinical benefit of CSA17, 18.
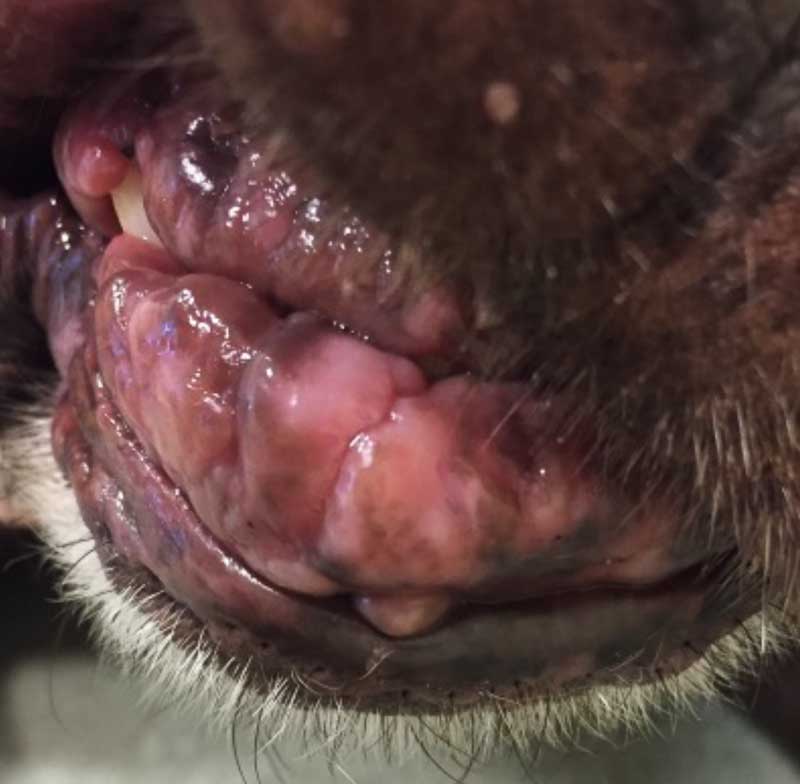
Gingival hyperplasia (Figure 4) and excessive hair growth can occur in a subset of patients, and this usually resolves on cessation of treatment. CSA utilizes P450 cytochrome enzyme system for metabolism, and known drug interactions exist which prescribing vets should be familiar with in pets on CSA.
Rarely, opportunistic infections can develop, and so owners should be advised to monitor for development of single or multiple nodules, as well as draining tracts.
No evidence exists to suggest regular biochemistry/haematological analysis and urinalysis is required in patients on long-term CSA, unless a clinical suspicion exists. Long term, reducing frequency of dosing rather than total dose (for example, giving medication on five days per week) is usually the most effective way to taper treatment, which can also lower the cost.
LKV is a caninised IL-31 monoclonal antibody which acts to bind and neutralise IL-31, a major pruritogenic cytokine. The author often describes use of LKV as line fishing for a single type of fish when describing its mode of action to clients.
LKV has been reported to reduce pruritus by 60% and lesion score by around 45%19. A study found when given proactively to house dust mite-sensitised Maltese-beagle cross-breeds (n=4) prior to application of house dust mite extract, LKV prevented expected pruritus, but not lesion development20. The same study also followed a group of 21 client-owned dogs with spontaneous atopic dermatitis who were receiving monthly or bimonthly LKV, and followed them to time to flare (TTF) after cessation of other antipruritic medications20.
This study found variance in TTF from only 3 days to 718 days, with a median TTF of 63 days, and this likely reflects different endotypes of CAD20.
A follow-up study by the same group in a colony of Maltese-beagle cross-breeds with spontaneous CAD, challenged with house dust mite extract, noted that sole IL-31 inhibition with LKV was insufficient to prevent expression of other pro-inflammatory mediators21.
A blinded randomised clinical trial assessing LKV (n=142 dogs) and CSA (n=132 dogs) found non-inferiority of LKV to CSA in terms of itch score, but lesion score (CADESI-03) was improved slightly in CSA patients (56.86%) versus LKV (54.17%)22.
The difference in lesion score is highly likely associated with CSA having recognised broader anti-inflammatory effects. Taken altogether, these studies illustrate why LKV is not effective in all CAD patients and that duration of activity can vary.
LKV could be considered appropriate in some patients for reactive treatment where no obvious inflammation or erythema is present, but where significant pruritus is reported. However, non-visible inflammation within the skin can be present, so GC or OCA may be more appropriate, and LKV reserved for proactive treatment.
If patients do not respond to two injections of LKV at appropriate four-week intervals, and no evidence exists of mitigating factors including secondary infection, then LKV is unlikely to successfully manage clinical signs and alternatives should be considered. Side effects are rarely seen in patients treated with LKV; those reported include vomiting, diarrhoea and hypersensitivity.
Topical products can be effectively utilised to manage clinical signs in patients with localised disease.
Topical GCs such as hydrocortisone aceponate can be used in both reactive and proactive stages of CAD. The calcineurin inhibitor tacrolimus can be applied to local areas; however, again owing to its delay in efficacy due to the mechanism of action, it is appropriate only in proactive treatment.
Proactive management of the allergic component of CAD
Allergen-specific immunotherapy (ASIT) is the only therapeutic shown to help manage the allergic component of CAD. This treatment takes three to six months to start to become effective and, therefore, is unsuitable for initial management of disease.
ASIT comes in three main forms: intralymphatic (ILIT), sublingual (SLIT) and subcutaneous (SCIT). ASIT acts to induce production of T regulatory cells by IL-10 and transforming growth factor ß23.
Formulation of ASIT is dependent on the results of allergen testing, either by allergen serology or intradermal testing (IDAT). Testing is not a diagnostic tool and should only be performed following discussion with the client about the success rate of ASIT, as well as the modes of administration.
Success of ASIT is unaffected by the mechanism of identification of allergens, but on the patient history. Decision making on allergens for inclusion in ASIT is based on the patient history, as well as known cross-reaction and co-sensitisations between allergens.
Each bottle of ASIT contains a set concentration of allergen; therefore, the more allergens included within a bottle, the lower the concentration of each individual allergens, and this may be insufficient to induce tolerance.
ASIT can be given subcutaneously (SCIT), initially in the vet clinic to monitor for rare adverse effects before the client can then administer this treatment at home or sublingually (SLIT). SLIT is more labour intensive than SCIT, and this is usually given twice daily with a lower concentration of allergens including in the solution before being titrated up.
ILIT can be performed in clinic without ultrasound guidance, with one injection (0.1mls) given every four weeks for six months before changing to SCIT protocol and often giving 1ml every four weeks24.
Pros and cons to each of these forms of ASIT exist, with a recent review by Mueller discussing these25. ASIT is an ever-evolving field, and further advancements will come over the next few years.
Skin care is a core part of management of atopic dermatitis in human patients with this condition. However, accessing our patient’s skin through the coat can be tricky. This is an area currently under research in CAD – especially as it is has not been determined if the noted changes are caused by or a consequence of CAD.
Current recommendations are to use a combination of omega-3 and omega-6 fatty acids; the optimum ratio is not known. Most premium dog foods with a dermatology focus are well fortified with these fatty acids.
Patients with CAD often develop secondary infections associated with inflammation of the skin development, followed by dysbiosis and then subsequent infection. Identification and management of these infections is out with the remit of this article.
Now that we have looked at the mechanism of action of the therapeutics available on the market, and whether they should be used for reactive versus proactive treatment, the following scenario should help with patients that companion animal vets see regularly.
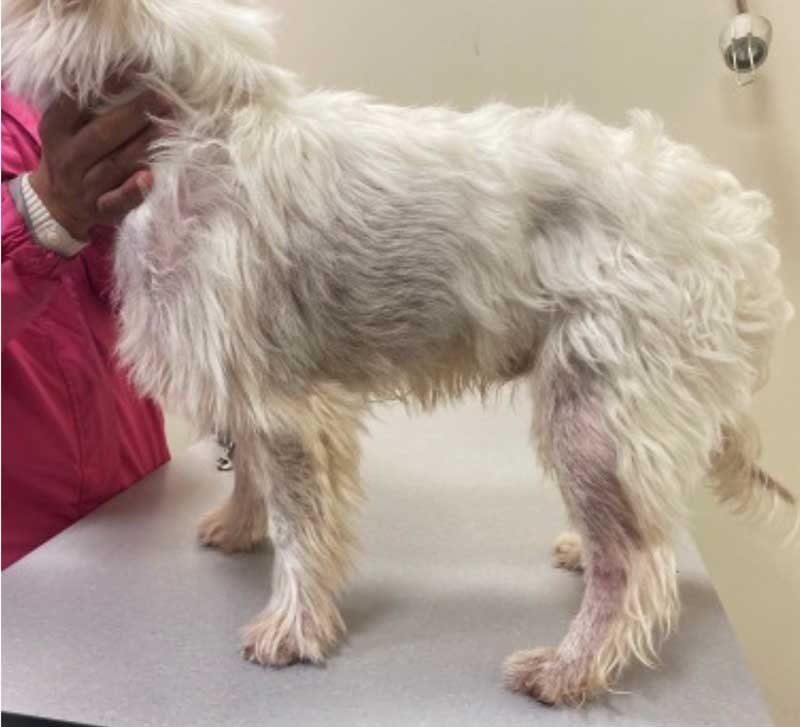
A three-year, six-month-old male, Fergus is a neutered West Highland white terrier with CAD (Figure 5). He has previously been managed with LKV and more recently OCA; however, itch has progressed.
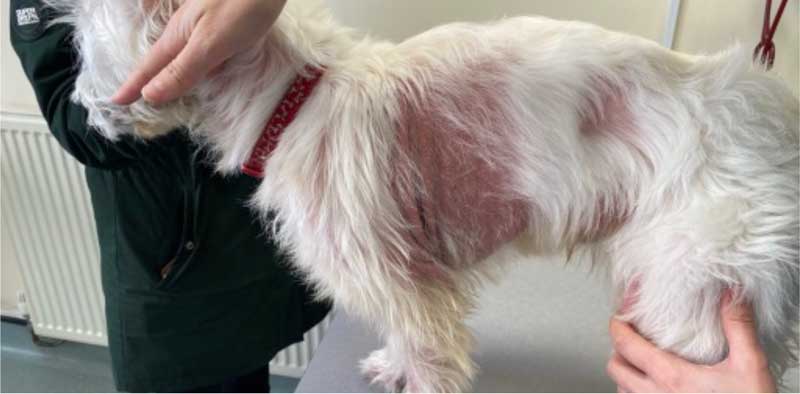
He has recently developed significant malodour and alopecia. His itch score is 8 out of 10 using the Visual Analog Scale26, and predominantly focused on the axillae, thorax, flanks and caudal ventral abdomen.
Mild Malassezia species overgrowth is visible on cytology from the skin, with no evidence of ectoparasites on skin scrape. Trichogram showed barbering of the hairs consistent with self-induced trauma associated with both pruritus and mild Malassezia species overgrowth.
Given the level of significant inflammation characterised by noticeable erythema, as well as the high itch score, reactive treatment with glucocorticoids – in this case 1mg/kg prednisolone for two to three weeks prior to tapering – was chosen.
The Malassezia species overgrowth was also treated with topical chlorhexidine.
Following this, OCA was reinstated and utilised alongside topical GCs to manage clinical signs (Figures 6 and 7). ASIT was also started in the form of ILIT following IDAT, and this combination is working well.

If Fergus has further episodes of significant inflammation, then GCs can be utilised as reactive treatment to induce remission before considering changing to CSA.
Due to the mechanism of action and the delay in time to efficacy, the author uses a short two-to-three week tapering course of prednisolone at the same time as starting CSA treatment.
CAD is a common, chronic, pruritic and inflammatory skin disease that has an impact on both owners’ and patients’ quality of life, and affects approximately 10% of canine patients.
Multiple therapeutic agents, both systemic and topical, are available to manage this condition, and the choice of multimodal therapy should be individualised to the patient and client.
Recent licensing of a new JAK-inhibitor, ilunocitinib, for CAD in the US, Canada and Japan will give vets, pets and owners an additional therapeutic to consider in management of these patients.
CAD is an area of constant research in veterinary dermatology which will hopefully help improve the quality of life of our canine patients.
