1 Jun 2016

Figure 1. A thermal (burn) chest injury in a bulldog. The area is severely inflamed and later became necrotic.
Local infection and inflammation is a common finding in veterinary and human medicine, with treatments being routine and fairly predictable. Once this condition becomes overwhelming, however, the situation can quickly become more serious and life-threatening.
Systemic inflammatory response syndrome (SIRS) and sepsis are topics of much debate in emergency medicine. Patients with SIRS have significantly higher mortality rate percentage than those with local inflammation and, in sepsis, that percentage almost doubles. A study of dogs with severe bite wounds in 2014 showed 54.3 per cent of those admissions had SIRS. These patients had a 24.5 per cent mortality rate with 27.7 per cent going on to develop multiple organ dysfunction (Ateca et al, 2014).
It is vital VNs are able to spot signs of this condition and understand the implications. Prompt, aggressive treatment therapies are usually necessary to bring the situation under control. Both human and veterinary professionals are still developing strategies in treatment and monitoring to decrease the loss we can suffer in these cases.
Inflammation is a natural occurrence in the body that occurs in response to injury or illness.
Systemic inflammatory response syndrome (SIRS) is an exaggerated inflammatory condition that can start from a local source, yet becomes widely systemic. SIRS can result from infectious causes, such as pyothorax, intestinal rupture or open wounds, and non-infectious causes, such as pancreatitis, severe trauma or burns (Figure 1).
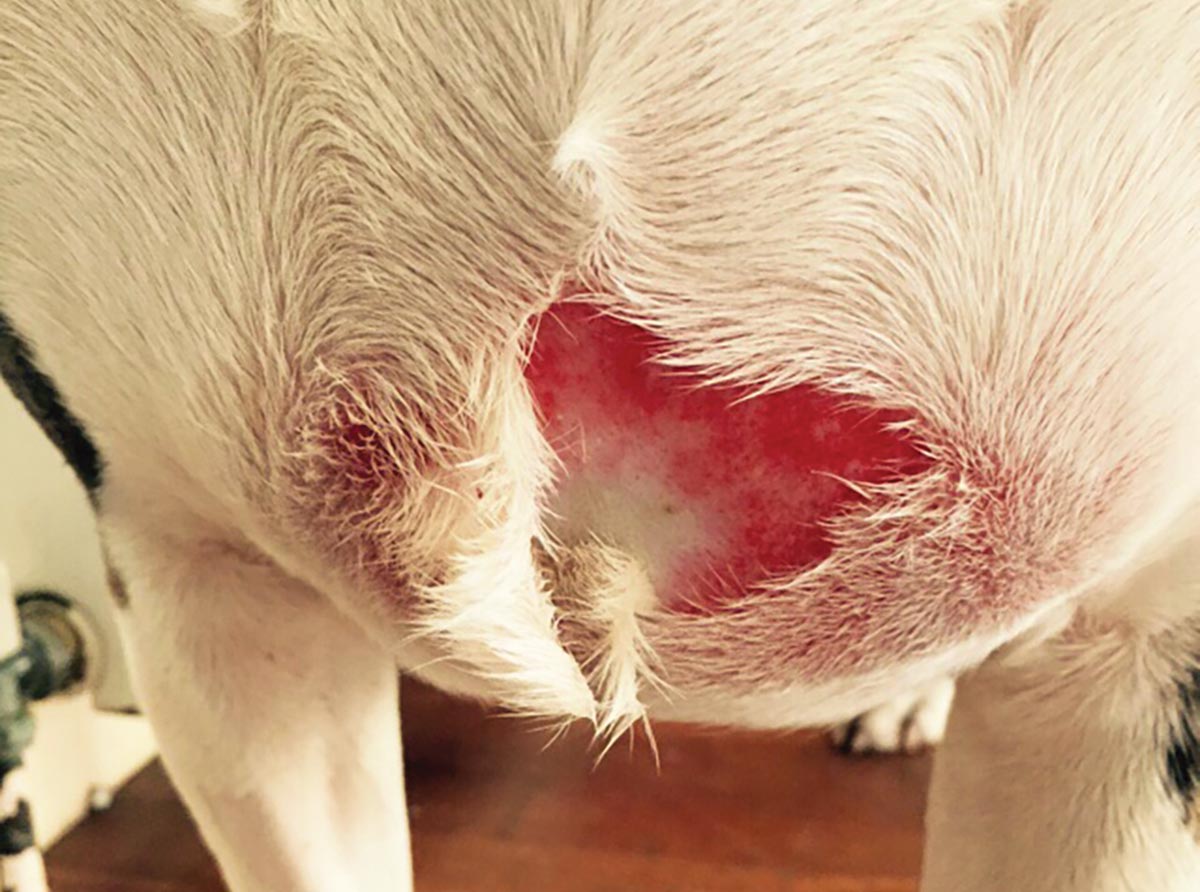
When infection is confirmed, SIRS is then known as sepsis. In severe cases, the condition can lead to multiple organ dysfunction syndrome and even death, and there is a 20% increase in mortality for each organ that fails.
In human medicine, 40% to 70% of intensive care unit admissions have SIRS or sepsis. The prognosis can be quite poor, with 7% fatality in SIRS compared with 46% in septic shock. The same can be said in animals and we are continuously performing trials and research to decrease that percentage.
When tissue is damaged in trauma, or a bacterial or viral invasion enters the body, three major changes occur, in an effort to destroy, wall off or dilute the agent and affected tissue:
These actions are pro-inflammatory and compensatory secondary actions, called anti-inflammatory, eventually restore equilibrium – things must be balanced to prevent suppressing the immune system and increasing risk to further infection. However, once the inflammation becomes systemic, affecting organs away from the initial source, this compensatory system fails and it is the failure of this system that leads to catastrophic events.
Inflammatory mediators from epithelial cells and acute phase proteins (APPs, from the liver) continue to be released into the blood. Unfortunately, as well as being beneficial, some are toxic and damaging to epithelial cells.
Increased vascular permeability allows leaking of protein-rich fluid, giving rise to hypoalbuminaemia, and the anticoagulant system can be affected, increasing the risk of hypercoagulation and microthrombi (small clumps of fibrin, platelets and red blood cells) formation. In severe cases, disseminated intravascular coagulopathy will follow, showing as surface petechiation (little blood clots in the skin).
Tissue bleeding will ensue once clotting factors have been diminished and platelets reach three high power fields (HPFs) to four HPFs. The patient eventually goes into distributive shock (non-infectious) or septic shock (if infection is confirmed) due to mass vasodilation, which causes hypoperfusion. Myocardial dysfunction can be seen in SIRS and sepsis caused by ischaemia. In the case of fluid volume loss, hypovolaemic shock can also be running at the same time.
The organ most vulnerable in dogs is the gastrointestinal (GI) tract. The mucosal layer is disrupted, leading to bacterial translocation. In cats, the organ at risk is the lungs, with changing permeability allowing protein to secrete into the alveolar sacs. Reduced oxygen uptake due to impaired gaseous exchange leads to acute respiratory distress syndrome.
What are the symptoms of SIRS?
Patients are said to be showing signs of SIRS if they have at least two out of the following four symptoms accompanying their illness:
Dogs may also have injected (red) mucous membranes and bounding pulses (hyperdynamic response). Cats, however, can present with bradycardia and pale mucous membranes instead. They are more prone to hypotension, hypoglycaemia and increased total bilirubin, too.
Pyrexia is common and stops bacteria consuming iron necessary for them to live, plus many viruses are also heat-sensitive. Active cooling is counterproductive; however, temperatures reaching 41.6°C will cause cell damage. It should, therefore, start at 41°C.
Efforts to reinstate sufficient tissue perfusion to organs must be aggressive. This is your main goal and first line of treatment. If you treat the systemic factor and not the cause, you will keep going in circles, so your second goal is to target the cause.
You should work within a maximum six-hour timeframe to reach your end goals – evidence exists that longer timeframes decrease survival. It is best to place two IV catheters (jugular ideally) to prevent multiple venepuncture for tests and so two fluids can run at the same time, if necessary.
Isotonic crystalloids are your first choice for circulatory support as they are balanced solutions (excluding 0.9% saline) that can freely cross the capillary and are a similar concentration to blood.
Hartmann’s solution is preferred as it is the least acidic and helps in the case of metabolic acidosis from hypoperfusion. It quickly redistributes within the first 30 minutes to 1 hour, with only 25% of the volume administered remaining intravascularly. Saline 0.9% can increase risk of hypernatraemia, so is not always appropriate in large volumes.
Dogs do better with rapid rate of fluid administration 60ml/kg/hr to 90ml/kg/hr. However, cats are not as tolerant and do better with small volume 10ml/kg to 20ml/kg boluses to effect. The patient should be re-evaluated every 30 minutes to 1 hour and treatment plans continued or changed.
Take caution in volume-intolerant patients, such as cardiac and kidney disease or lung and brain injury, as this could be counterproductive. Once out of shock, continue fluids to replace the deficit over the next 24 hours, taking into consideration ongoing losses, such as vomit, diarrhoea and wound exudate (Figure 2). If crystalloids alone are not enough to improve perfusion and return mean arterial pressure (MAP) to around 70hg to 120hg, then you may have to add a colloid.

Synthetic colloids help pull fluid into the intravascular space to increase blood pressure and tissue perfusion. Their bigger molecules cannot freely pass capillaries, so are usually broken down by enzymes, absorbed or excreted through the kidney.
Three types exist: hydroxyethyl starch (HES), gelatins or dextran 70. HES is the most commonly used, comes in three molecular weights and lasts longer than dextran 70. It can be given in bolus to effect or constant rate infusion (CRI) using a maximum of 20ml/kg/24hr (10ml/kg/day to 40ml/kg/day in pentastarch).
The disadvantage is its risk of adverse reactions, even within the safe dose range. It can cause anaphylaxis, platelet dysfunction, coagulopathy disorders and acute kidney injury. It is advised to hold off using it unless the amount of isotonic crystalloids required may overinfuse the patient, or if a capillary leak condition is present, which, in SIRS, is common.
Hypertonic saline (7.2% sodium chloride) is a safer type of volume expander, but is fairly short acting (around 30 minutes) because of its rapid breakdown into free water. However, following it with a bolus of colloid can prolong its effects. It is an aggressive fluid and is given in a 2ml/kg to 4ml/kg bolus in cats and 4ml/kg to 7ml/kg in dogs over 15 minutes – the equivalent of giving 60ml/kg to 90ml/kg of isotonic crystalloids.
Unfortunately, it is contraindicated in existing hypernatraemia and cannot be used in dehydrated patients before a sufficient interstitial and intracellular fluid reserve is established. It is also not advised in active bleeding or pulmonary contusion cases where aggressive fluid can cause a fatal haemorrhage from damaged vessels.
These patients should only be resuscitated to a MAP of 60hg for this reason. An ECG should be attached when giving hypertonic saline as it can cause cardiac dysrhythmias if given too quickly.
If fluid therapy support is still failing to restore blood pressure and perfusion then drug intervention may be warranted. It can be difficult to tell whether hypotension exists because of a vasodilatory disorder or other causes.
It has been known septic patients can have a deficiency in vasopressin, leading to systemic vasodilation. Pros and cons exist for this type of therapy (Table 1). All the drugs are administered as a CRI and close ECG monitoring is a must.
| Table 1. Comparison in hypotensive drugs | |||
|---|---|---|---|
| Drug | Type | Uses in systemic inflammatory response syndrome/sepsis | Disadvantages |
| Vasopressin | Posterior pituitary hormone | Can be used in conjunction with noradrenaline. Should only be used in dire septic situations where other vasopressors have failed. | Has shown higher mortality in sepsis trials. Can constrict myocardial arteries – do not use in heart disease. |
| Adrenaline | Endogenous catecholamine | Second line drug of choice. If noradrenaline fails, it can be added in or used as its substitute. Increases heart rate, contraction and output. Relaxes bronchial smooth muscle and increases glycogenolysis. | Increases work and oxygen demand of the heart. Tachycardia and possible ventricular fibrillation and other arrhythmias. |
| Norepinephrine | Catecholamine | First line drug of choice. Increased mean arterial pressure through vasoconstriction with minimal effects on the heart. | Can increase, decrease or unchange the heart contractility, depending on the current circulating volume. May increase heart rate. |
| Dopamine | Endogenous catecholamine | Low dose = vasodilation with increased organ perfusion, cardiac output and renal blood flow. High dose = increased heart rate and contraction, vasoconstriction. Releases noradrenaline. Used in hypodynamic states seen in shock. | High doses can reduce renal blood flow and cause tachycardia with or without arrhythmias. Should only be used in cases with bradycardia or low risk of tachyarrhythmias. |
| Dobutamine | Dopamine analogue | Has reduced effect on cardiac contractility and vasculature so good for heart failure. Used for low cardiac output with increased filling when already on vasopressors. Treats hypoperfusion despite normal mean arterial pressure and blood volume. Does not release noradrenaline. | Tachycardia with marked increase in systolic pressure. Possible ventricular arrhythmias. |
| Phenylephrine | Selective adrenergic agonist | Used as a last resort if shock still prevails despite whether two or more vasopressors with vasopressin have failed. Can be used if noradrenaline has caused a serious cardiac arrhythmia. Peripheral vasoconstriction with increased blood pressure and longer circulation time. | Can raise blood pressure to the expense of vital organ perfusion. Tachycardia or reflex bradycardia. |
Use of blood transfusion in critical patients is common practice and largely available in dogs. Fresh frozen plasma (FFP) transfusion is a good natural colloid therapy that has essential proteins with anti-inflammatory properties, electrolytes and replacement clotting factors. It has also been shown to improve epithelial integrity in patients.
Large volumes of plasma would be required to replace proteins specifically, so the risk of circulatory overload (pulmonary and peripheral oedema or pleural effusion) is increased – especially in cats, which are more susceptible.
Transfusion rates will need to be lowered in patients with cardiac or renal disease, increasing the length of time for volume completion. As plasma should not be at room temperature for more than four hours, it is necessary to administer their full volume in two separate boluses. Watch out for hypothermia if giving large volumes not warmed prior to use.
Cats are more likely to become anaemic during illness (haemolysis or decreased production and loss) than dogs and any patient with a PCV less than 20 is a potential candidate for whole blood (WB) or packed red blood cell (PRBC) transfusion.
Cat blood is harder to store, so a full blood transfusion is often required instead of separate products. Cat blood must be typed as they have naturally occurring antibodies. The rough rule of thumb is 2ml of WB per kg infusion gives a rise in PCV of 1%.
A risk of hypocalcaemia also exists as citrate is the most common anticoagulant used in collection and chelates (bonds) to calcium ions removing them from the blood stream.
Oxyglobin (bovine haemoglobin) can be used if WB or PRBCs are unavailable for increasing oxygen delivery to tissues. It shifts the majority of oxygen carriage to plasma, but is not licensed in cats and is a strong colloid, so beware of circulatory overload.
A natural colloid is albumin and human versions are available that are tolerated fairly well. They have much smaller molecules, so it is not the most effective at raising blood volume. It can only be given once because the immune system develops antibodies against it in the days post-transfusion. It is a good product where significant hypoalbuminaemia prevails for its various important roles throughout the body.
Critical patients are at higher risk of malnutrition. The metabolism is altered, so nutritional support is essential and proven to increase long-term prognosis. Prolonged anorexia can lead to ileus, loss of mucosal integrity and immunity of the GI tract. The body starts synthesis of its own tissues, ending in an increased catabolic state.
Cats are prone to hepatic lipidosis from fat breakdown causing liver dysfunction. A nutritional assessment and feeding plan should be made to suit the individual’s needs, but cannot begin until it is haemodynamically stable.
Enteral feeding is preferred over a glucose drip or total parenteral nutrition. A feeding tube should be placed, if possible, so support can
start as soon as possible. Feeding tubes must not be placed in the event of facial or upper GI tract injury.
A higher oxygen demand exists of all tissues, which will persist for a time even after perfusion is restored. Oxygen therapy is, therefore, strongly advised as tissues will be in a state of oxygen debt. Sufficient pain relief is an important part of shock treatment as pain is pro-inflammatory. It also causes anorexia, elevated heart rate, withdrawn or aggressive behaviour and prolongs shock.
Early treatment with broad-spectrum antimicrobials in sepsis has improved prognosis. They must be broad enough to cover all pathogens until the agent is identified, and each hour that goes by without them increases mortality. An increased risk of stress ulcers on the GI tract is present, so proton pump inhibitors, such as omeprazole, or H2 blockers, such as ranitidine, should be added to the plan.
Your end goals should be to return vital signs to within the normal range, improve demeanour and behaviour, have urine production to at least 0.5ml/kg/hr to 2ml/kg/hr and a blood pressure systolic higher than 80, with MAP 60 and above. In the critical stage, recordings of these should be as frequent as every 15 minutes.
If the patient does not tolerate a physical check then record as much as you can from outside the kennel and only do physical checks every 30 minutes. You can slowly reduce your recordings as the patient improves to every 30 minutes, 40 minutes, then hourly, until stable.
Carry out a full blood panel, such as biochemistry, haematology with a fresh blood smear and manual PCV/total protein (TP), blood gases and lactate. This can provide lots of information used to tailor the treatment plan. Blood glucose and manual PCV/TP should be done frequently due to changes that occur in circulatory resuscitation.
Hypoperfusion reduces oxygenation and build-up of lactate in the tissues leads to lactic acidosis. During correction of perfusion, lactate levels should reduce if treatment is adequate. We should check the lactate hourly and change the plan accordingly. If no reduction occurs, despite efforts, it carries a poor survival prognosis.
VNs spend a lot of time with sick patients and can be the first to spot changes. The author believes if VNs know the signs to look out for, and in what conditions to expect them in, rescue attempts could be started sooner. She has seen success and loss through SIRS and sepsis in various conditions. Recognition, timing and aggression of treatment seem to aid prognosis.
Research in dog models has shown plasma C reactive protein (CRP), interleukin-6 (IL6) and procalcitonin may be useful blood biomarkers in the severity of the inflammation condition, whether local or systemic, if antimicrobials are indicated and, on repeat testing, could aid in the response to treatment.
CRP is increased in sicker dogs and those with decreased levels after treatment has increased survival rates. High levels of IL6 also show higher percentage of mortality. However, studies are still underway in this field.