30 Jun 2021

Acute haemorrhagic diarrhoea syndrome.
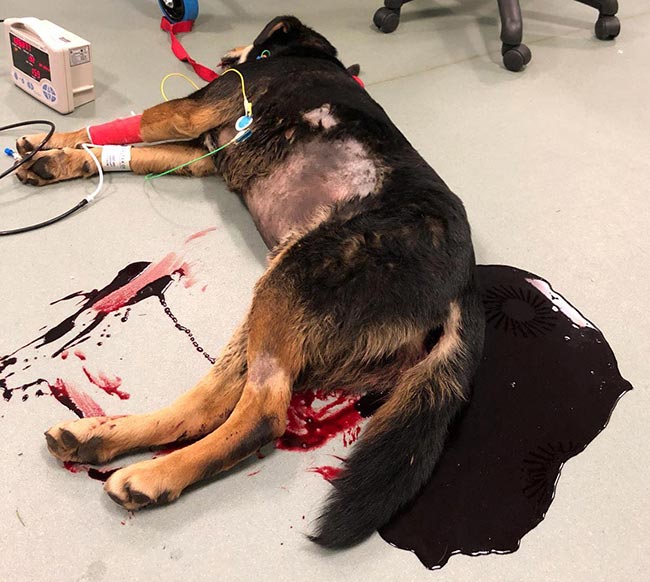
Acute haemorrhage diarrhoea syndrome (AHDS), previously known as haemorrhagic gastroenteritis, is a common emergency presenting to veterinary practice.
This syndrome commonly affects young to middle-aged, small-breed dogs, and is characterised by the peracute onset of vomiting and diarrhoea with blood, which can lead to life-threatening dehydration and hypovolaemia.
Dogs with AHDS have marked haemoconcentration, with PCVs often in excess of 60%, but lack the concurrent increase in total proteins (Trotman, 2009). The majority of dogs will improve in the first 48 hours, but some can require extensive hospitalisation and care, which can prove challenging.
Despite considerable research, the underlying cause for AHDS remains unknown. Suggested aetiologies include roles for Clostridium perfringens enterotoxin and Clostridium difficile toxin A/B; however, studies have failed to prove a consistent link and their role in AHDS remains controversial (Busch et al, 2014; Unterer et al, 2014).
Assessing the severity of AHDS can be performed using the canine AHDS activity index, as detailed in Table 1 (Unterer et al, 2011) although this remains to be validated for prognosis.
| Table 1. The canine acute haemorrhagic diarrhoea syndrome activity index (Unterer et al, 2011) | ||||
|---|---|---|---|---|
| Score | 0 | 1 | 2 | 3 |
| Appetite | Normal | Mildly decreased | Moderately decreased | Severely decreased |
| Vomiting | No | Once a day | 2-3 times a day | More than 3 times/day |
| Faecal consistency | Normal | Slightly soft | Very soft | Watery |
| Defecation frequency | Normal | 2-3 times/day | 4-5 times/day | More than 5 times/day |
| Dehydration | No | <5% | 5-10% | >10% |
This article will cover the diagnostic approach and management of dogs with AHDS.
A thorough history and clinical examination are the first steps for investigating a suspected case of AHDS. Similar clinical signs can be caused by dietary indiscretion, toxin ingestion or infectious diseases such as canine parvovirus, canine enteric coronavirus and Giardia species.
Findings on clinical examination such as cachexia, cutaneous evidence of coagulopathies, marked cranial abdominal pain or organomegaly may increase suspicion of other clinical disease processes that can cause haemorrhagic diarrhoea.
Some causes of haemorrhagic diarrhoea can be rapidly investigated with clinical examination, history taking or rapid in-house tests. Initial tests would include PCV and total solids, complete blood count and a blood smear, serum biochemistry, with electrolyte and venous blood gas analysis as well as coagulation testing performed if available.
AHDS is a diagnosis of exclusion; however, abdominal ultrasound and endoscopy can be used to visualise the characteristic ulcerative lesions in the small intestine (Unterer et al, 2014).
Other differentials can be ruled out using further testing such as abdominal imaging, faecal analysis and specialised laboratory tests (Table 2).
| Table 2. Available diagnostics to rule out differential diagnoses (Maddison, 2015) | |||
|---|---|---|---|
| Differential diagnosis | Differential diagnoses | Available in-house diagnostics | Available external diagnostics |
| Bacterial infection | • Campylobacter species • Escherichia coli • Salmonella species |
• N/A | • Faecal culture • PCR |
| Viral infection | • Parvovirus • Canine enteric coronavirus • Canine distemper virus |
• SNAP Parvo test | • Faecal PCR (available for all three viruses) |
| Parasitic infection | • Giardia species • Angiostrongylus vasorum (lungworm) • Trichuris vulpis (whipworm) • Ancylostoma caninum (hookworm) |
• SNAP Giardia test • Angio Detect • Faecal microscopy |
• PCR • Zinc sulfate flotation • Faecal microscopic exam • Baermann technique |
| Inflammatory disease | • Inflammatory bowel disease • Immune-mediated thrombocytopenia* • Pancreatitis |
• Endoscopy and biopsy • Ultrasound • Blood smear evaluation • SNAP cPL • Quantitative CPLi |
• Histopathology • Complete blood count and smear evaluation • Quantitative CPLi assay |
| Metabolic or secondary gastrointestinal | • Hepatobiliary disease • Hypoadrenocorticism |
• Biochemistry • SNAP cortisol • Quantitative basal cortisol |
• Bile acid stimulation test • Basal cortisol • Adrenocorticotropic hormone stimulation test |
| Neoplasia | • Pancreatic adenocarcinoma • Lymphosarcoma/lymphoma |
• Ultrasound • Endoscopy • Exploratory laparotomy |
• Histopathology • Cytology |
| Obstruction | • Foreign body • Intestinal neoplasia |
• Radiography • Ultrasound • CT |
• Radiography • Ultrasound • CT |
| *Immune-mediated thrombocytopenia can be triggered by many of the other conditions listed here. | |||
In acutely sick dogs with AHDS, marked dehydration can lead to hypovolaemic shock.
When signs of hypovolaemia (tachycardia, hyperdynamic or poor peripheral pulses, pallor) are appreciated, initial crystalloid fluid therapy with boluses guided by blood pressure measurement and improvement of cardiovascular parameters is recommended.
When hypovolaemia is not appreciated or has been corrected, a clinical assessment of dehydration should be made (Table 3) and a plan to correct this should be initiated. It should, however, be remembered that clinical signs of dehydration are widely variable and often inconsistent between patients (Boag and Hughes, 2007).
| Table 3. Clinical assessment of dehydration (Boag and Hughes, 2007) | |
|---|---|
| Clinical signs | Estimate of dehydration in percentage of bodyweight |
| Normal | Below 5% |
| Dry mucous membranes only | 5% |
| Reduced skin turgor | 6-8% |
| Increased heart rate* | 8-10% |
| Weak pulses* | 10-12% |
| Collapse and shock* | 12-15% |
| *Are signs associated with hypovolaemia rather than dehydration per se. | |
Once an estimate of dehydration has been made then the patient’s fluid deficit can be calculated using the following formula:
(Bodyweight × estimate of dehydration) / 100 = fluid deficit in litres
Fluid deficits should be corrected over 12 to 48 hours in addition to allowing for estimated maintenance requirements and ongoing losses.
In most circumstances, a balanced electrolyte isotonic crystalloid, such as Hartmann’s solution (compound sodium lactate), would be an appropriate first choice.
In some patients, re-establishing and maintaining euvolaemia with crystalloids alone can be challenging due to hypoproteinaemia, third spacing and ongoing fluid losses. In these patients, products such as artificial colloids or frozen plasma can be considered for their crystalloid-sparing effects or vasopressors, such as noradrenaline, used to maintain arterial blood pressure.
Abdominal pain is a common clinical sign associated with patients with AHDS and is often underappreciated in patients whose mentation is affected. Multimodal analgesia should be administered, including opioid pain relief such as methadone or buprenorphine.
Using pain scores to guide the use of analgesia can help select an appropriate agent, dose and time frame to prevent both the underuse and overuse of opioid analgesia, both of which can affect improvement and prognosis.
Most veterinary surgeons would instinctively avoid the use of NSAIDs in animals with gastrointestinal signs; however, other agents such as paracetamol may have a role to play in the management of abdominal discomfort. The topic of analgesia is well covered in “Companion animal analgesia” (Walsh, 2019).
Antiemetic medication plays a vital role in the management of AHDS. Nausea is both considered a welfare concern and will slow improvement in patients that are hyporexic or anorexic.
Drugs such as maropitant and ondansetron can be used as antiemetics to control nausea in patients with AHDS. Some evidence shows that maropitant, as a neurokinin-1 inhibitor, acts as a visceral analgesic in addition to its central antiemetic properties (Sullivan et al, 2011).
Patients that develop ileus can be treated with prokinetics, such as metoclopramide, in the form of either boluses or continuous rate infusions. Where haematemesis has been noted, proton pump inhibitors such as omeprazole or pantoprazole can be considered.
Early enteral nutrition is vital for improving motility and intestinal cell healing. Enterocytes on the villi draw nutrition from the intestinal contents, and so enteral nutrition is vital for intestinal repair and to prevent villous atrophy. (Eirmann and Michel, 2009).
Where possible, antiemetics and prokinetics should be used to reduce nausea and encourage independent feeding. When this approach is not successful, naso-oesophageal or nasogastric tubes are a cost-effective approach to providing nutrition. Nasogastric tubes have an advantage in patients that have a buildup of gastric fluid contributing to discomfort or nausea as this fluid can be drained regularly (Eirmann and Michel, 2009).
Feeding can be initiated in a gradual buildup to full resting energy requirements and can be given in bolus or as a “trickle feed” using a syringe driver.
Providing nutrition for critically ill patients has been discussed in a previous article, “Managing food refusal in critically ill patients” (Weeth, 2016).
Historically, antibiotics such as metronidazole and amoxicillin/clavulanic acid have been used for a multitude of reasons, including the proposed immunomodulatory effects of metronidazole and treatment of suspected bacterial infection. Repeated clinical studies have failed to demonstrate any significant benefit to the administration of antibiotics for dogs with AHDS (Unterer et al, 2011; Ortiz et al, 2018).
Patients with proven infection should be treated with an appropriate antibiotic with culture and sensitivity as guidance. Patients with demonstrated neutropenia should be treated with an appropriate broad-spectrum antibiotic such as amoxicillin/clavulanic acid.
Patients with AHDS should be closely and frequently monitored to ensure their fluid demand is appropriately met. Monitoring of PCV, total solids and electrolytes every 12 to 24 hours is recommended to monitor fluid therapy and protein levels. Due to a combination of loss and reduced intake, many patients with AHDS develop electrolyte disturbances such as hypokalaemia, which should be corrected by supplementation in fluid therapy.
Repeated abdominal point of care ultrasound can be used to monitor for third spacing of fluid occurring secondary to hypoproteinaemia. Assessment of intestinal peristalsis with the same technique can also be useful in decision-making for prokinetic use and gastric drainage to relieve nausea.
Thoracic point of care ultrasound can be used to assess for concurrent cardiac disease and for signs of “wet lung”, which may raise concern for aspiration pneumonia (Hamel and Berry, 2018).
AHDS is a common emergency condition seen in veterinary practice. Fluid therapy, analgesia, protection of the gastrointestinal tract and nutrition provide the cornerstones of a good therapeutic plan for these patients. Care should be taken to rule out other differential diagnoses – especially those needing specific treatment. With appropriate supportive care and monitoring, dogs with AHDS have an excellent prognosis.