6 Oct 2020

A common presentation for first-opinion and emergency practice is the acute abdomen; abdominal pain frequently presents alongside lethargy, inappetence and vomiting.
Canine-specific pancreatic lipase (cPL) is often used as part of the initial investigations as pancreatitis is a significant differential diagnosis. While a negative result excludes pancreatitis, clinicians must remain aware that an abnormal cPL does not confirm this condition and should be interpreted alongside the history, examination and other diagnostic tests to avoid missing another condition that may require specific treatment.
Acute abdominal pain in a dog – alongside lethargy, anorexia and vomiting – is a frequent presentation to first opinion and emergency practice.
Definitive diagnosis can be challenging due to the wide differential diagnoses. As acute pancreatitis is a common cause of this presentation, a desire has existed for convenient biochemical tests to confirm this diagnosis, of which the canine-specific pancreatic lipase (cPL) is the most sensitive and specific (Trivedi et al, 2011).
However, an over-reliance on this test in isolation without consideration for secondary causes of an abnormal cPL may delay diagnosis and specific treatment for other causes of this presentation. Implications also exist for the future of the patient due to the long-term dietary changes likely to be recommended following a diagnosis of pancreatitis.
This article will review evidence-based interpretation of this test and the use of it as part of a diagnosis of pancreatitis, avoiding pitfalls that may lead to missing underlying disease.
No single gold-standard clinical test to diagnose acute pancreatitis exists. A diagnosis can only be reached by a clinical picture, combining signalment, history, physical exam, blood tests and imaging.
Even histopathology is limited as focal lesions can be easily missed – it is generally used in studies only when the entire pancreas is examined postmortem (Neilson-Carley et al, 2011). When studies are evaluating potential diagnostic tests, a clinical picture is used to define the cases of pancreatitis (Cridge et al, 2018).
Traditional pancreatitis diagnosis included assessment of serum amylase and lipase; however, their utility is limited by poor specificity (Trivedi et al, 2011). Biochemical diagnosis has been improved by the commercial availability of cPL measurement (Trivedi et al, 2011). This was found to have improved sensitivity and specificity comparing cases considered to have a high or low likelihood of having acute pancreatitis (McCord et al, 2012) or histologically normal versus histologically confirmed pancreatitis (Mansfield et al, 2012).
The pancreatic lipase meeting these sensitivities and specificities is, hence, based on having a high index of suspicion for pancreatitis, whereas running the test indiscriminately would be expected to produce less reliable results.
A greater specificity has been found with a specific cPL (specPL) value of greater than 400; there is also a “grey zone” of 200 to 400 (Neilson-Carley et al, 2011). Darker or equal sample spots on the SNAP cPL in-house test (SNAP) are considered “abnormal” with a high level of accuracy (tested on spec values well below versus towards the top end of the grey zone; IDEXX Reference Laboratories, 2018).
As the sensitivity is considered high for pancreatic inflammation, a normal SNAP does quite reliably exclude pancreatitis, so cPL has the greatest accuracy when used as a rule-out.
An improved assay for detection of lipase using 1,2-o-dilauryl-rac-glycero-3-glutaric acid-(6’-methylresorufin) ester (DGGR) has been shown to have equivalence to cPL with the benefits of shorter turnaround and lower cost, with some labs including it in routine biochemistry panels.
However, it would not be expected to be any more accurate than cPL (Kook et al, 2014).
To provide more meaningful specificity data, a study examined cases of acute abdomen presentation in dogs and the likelihood of abnormal pancreatic lipase (Haworth et al, 2014).
They found that for the typical acute abdomen presentation, 40% of cases had a false positive SNAP, improved to 23% false positive with specPL. This also emphasised if making a diagnosis of pancreatitis the result should be confirmed by a laboratory with specPL, but even then the test cannot be relied on in isolation.
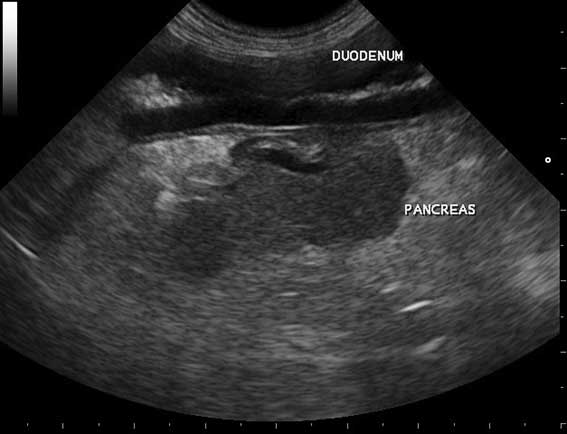
Common secondary causes were gastrointestinal (GI) foreign bodies, hepatic masses and multiple causes of septic peritonitis. When examining the pancreas by ultrasound, the landmarks include the duodenum (Figures 1 to 3); given the obvious physical proximity this shows it is unsurprising an upper GI foreign body would cause secondary pancreatic inflammation.
Interestingly, an unexpected finding was several cases with pancreatic carcinoma were SNAP and specPL normal.
In false positive cases, the increased cPL may arise because the primary pathology (such as duodenal foreign body) is causing secondary inflammation of the pancreas, which may or may not be contributing to the clinical presentation.
Another possible cause would be concurrent subclinical chronic pancreatitis exists – effectively an incidental finding and quite common (Watson et al, 2007). Remember not only Occam’s razor, but also Hickam’s dictum (Panel 1).
Occam’s razor (William of Ockham, 14th century) is a familiar concept in medicine that a single and most plausible diagnosis is the most likely, and would suggest an animal presenting suspicious for pancreatitis with an abnormal canine-specific pancreatic lipase is by far most likely to be pancreatitis.
However, this should be balanced with Hickam’s dictum (John Hickam MD): “A man can have as many diseases as he damn well pleases.” In this case, concurrent pancreatic inflammation may be secondary to a wide range of other abdominal pathology or incidental chronic pancreatitis.
Some other unrelated conditions have been associated with abnormal cPL levels. Dogs with intervertebral disc disease were found to have increased chance of abnormal cPL; this is suspected secondary pancreatitis due to autonomic disruption causing selective hypoxia of the pancreas (Schueler et al, 2018).
Spinal disease is particularly important, as the posture exhibiting lower spinal pain could be mistaken for abdominal pain and vice versa.
As many as 55% of dogs with hyperadrenocorticism have abnormal SNAP or spec results in absence of clinical pancreatitis (Mawby et al, 2014). The mechanism for this has not been elucidated yet and it is unclear if they are at increased risk of developing clinical pancreatitis in the future.
The question facing a clinician in general practice is likely to be if an individual case should have imaging (radiographs/ultrasound) as part of the initial investigations or if symptomatic/supportive treatment can be started “blind”.
Ideally, all cases of acute abdomen and/or suspected pancreatitis would have full investigation at the start, both to confirm the diagnosis and identify complications so the full spectrum of medical treatment can be confidently given. The author’s practice regularly sees examples with a final diagnosis of neoplasia, haemoabdomen or foreign bodies, so will readily recommend imaging early in investigations, even if the presentation is “typical” pancreatitis.
Frequently in general practice tests are appropriately prioritised based on individual presentation, owner willingness, financial limitations or sometimes the stability of the patient to undergo sedation or general anaesthesia. Applying this to a case of suspected pancreatitis, how can our initial investigations guide us on which individuals are a priority for further investigations such as diagnostic imaging and what would be helpful?
The signalment and history are the first diagnostic information we obtain for any case and are always the most important.
In the case of acute abdomen with suspected pancreatitis, consider the likelihood of pancreatitis being the primary cause and interpret an abnormal cPL level in this light. Better still, make this judgement prior to routinely running the test and decide if it will add useful information.
For example, a 10-year-old large crossbreed with no history of pancreatitis in its lifetime or recent diet changes has a much higher index of suspicion for neoplasia, compared to a young schnauzer that stole the remains of a family barbecue the day before developing symptoms. Even if both present with vomiting and abdominal pain.
For the former, cPL may be useful to exclude pancreatitis, but an abnormal level would not be diagnostic.
The physical examination is the next most important source of information. Some degree of abdominal pain is assumed, but to what degree? Is pallor present, suggesting anaemia and possible haemoabdomen? Is the patient showing signs of shock or systemic inflammatory response syndrome – tachycardia, tachypnoea, pyrexia or hypothermia? Is any palpable organomegaly present?
A number of changes on routine blood tests may guide us on the presence of a secondary cause. For example, neutrophilia with left shift or neutropenia may alert us to the possibility of a septic focus such as peritonitis. The lack of a stress leukemoid pattern (particularly a lack of suppressed lymphocytes) may alert the clinician to possible hypoadrenocorticism. Hypoglycaemia may be found in both of these conditions.
Anaemia would raise concern for haemoabdomen of various causes. Hepatic enzyme changes alert us to hepatic disease, as would hyperbilirubinaemia; these changes may also indicate complications of pancreatitis, such as bile duct obstruction.
An accurate diagnosis is much more likely if full differential diagnoses are considered for each abnormality and followed up as indicated.
If the facility is available, testing acid-base balance may reveal metabolic disturbances that may be more severe than expected for a ”simple” pancreatitis. Blood lactate measurement can be extremely useful in indicating more severe pathology such as tissue hypoxia or sepsis. C-reactive protein gives an indication of the degree of inflammation; it is most useful in this context as a monitor of treatment.
The use of this information will be guiding the initial supportive treatment and deciding whether this animal should have diagnostic imaging performed immediately, with possible progression to surgery for a secondary cause or a complication of severe pancreatitis such as abscessation.
Ultrasound is the most commonly used modality as it can allow visualisation of the pancreas itself and may identify the typical changes of a thickened hypoechoic pancreas with hyperechoic surrounding mesentery (Figure 4), as well as examining for evidence of the other causes of acute abdomen.
Radiographs are complementary and, if ultrasound isn’t available, can allow assessment for organomegaly, free fluid and indications of gastrointestinal foreign body.
If available, CT has been shown to provide the most comprehensive views of the pancreas, as well as identifying markers of increased severity more accurately, such as portal vein thrombosis with implications for prognosis (French et al, 2019).
If no other concerns exist and the index of suspicion for pancreatitis is high then supportive treatment for pancreatitis might be chosen over further investigations. If this approach is taken, the final information is response to treatment.
The patient should receive frequent ongoing reassessment, and if the response to treatment isn’t as anticipated (vomiting controlled, pain controlled, appetite returning) then the need for further investigation should be reassessed. An explanation to the owner at the time of initial investigations that secondary causes might exist and further investigation might be required is important to manage expectations.
Specific pancreatic lipase is a useful blood parameter as part of investigations for vomiting and abdominal pain. A negative result excludes pancreatitis; however, a positive should be interpreted with caution and owners fully informed of the implications – especially possible secondary causes:
A five-year-old male neutered springer spaniel was admitted for investigation of acute onset of vomiting.
There was no diarrhoea or previous history of gastrointestinal disease. The patient had been in a kennel all day and the risk of foreign body was considered low.
Clinical examination revealed only some discomfort around the stomach area. Haematology showed mild haemoconcentration and moderate neutrophilia causing leukocytosis. Biochemistry showed subtle changes in alkaline phosphatase and urea, and hypokalaemia. A SNAP canine-specific pancreatic lipase was clearly abnormal.
Secondary causes were discussed, but as the patient was stable and more comfortable with initial medical management, the decision was made with the owner to assess response to medical treatment before further investigations. IV fluid therapy was calculated to rehydrate and supplemental potassium chloride was added. Analgesia was started with buprenorphine and monitored by pain scores. Maropitant and ranitidine were given symptomatically.
On reassessment the following day, the vomiting had stopped, but the patient had no interest in food and still presented cranial abdominal discomfort despite increased analgesia, so further investigation was recommended. Repeat electrolytes confirmed normalisation of potassium, acid-base balance was normal and lactate was within normal range.
Abdominal radiographs showed some irregular gas in the intestines and a suspected fabric density foreign body (Figures 5 to 7).
Abdominal ultrasound confirmed a long irregular shadowing object in the small intestine including duodenum and some areas with fluid dilation (Figures 8 to 10). The shadowing largely obscured views of the pancreas, but areas that could be seen appeared normal.
Exploratory surgery revealed a fabric linear foreign body passing from the stomach through the duodenum and a large portion of the jejunum.
Significant damage to areas of the jejunum was present, necessitating a resection and anastamosis of this area. The pancreas was grossly normal.
Biopsies of the stomach and intestines revealed only the inflammation expected for a foreign body. A biopsy of the pancreas was histologically normal.
The patient made a full recovery and was discharged from hospital 72 hours later with no ongoing treatments required.