9 Oct 2017

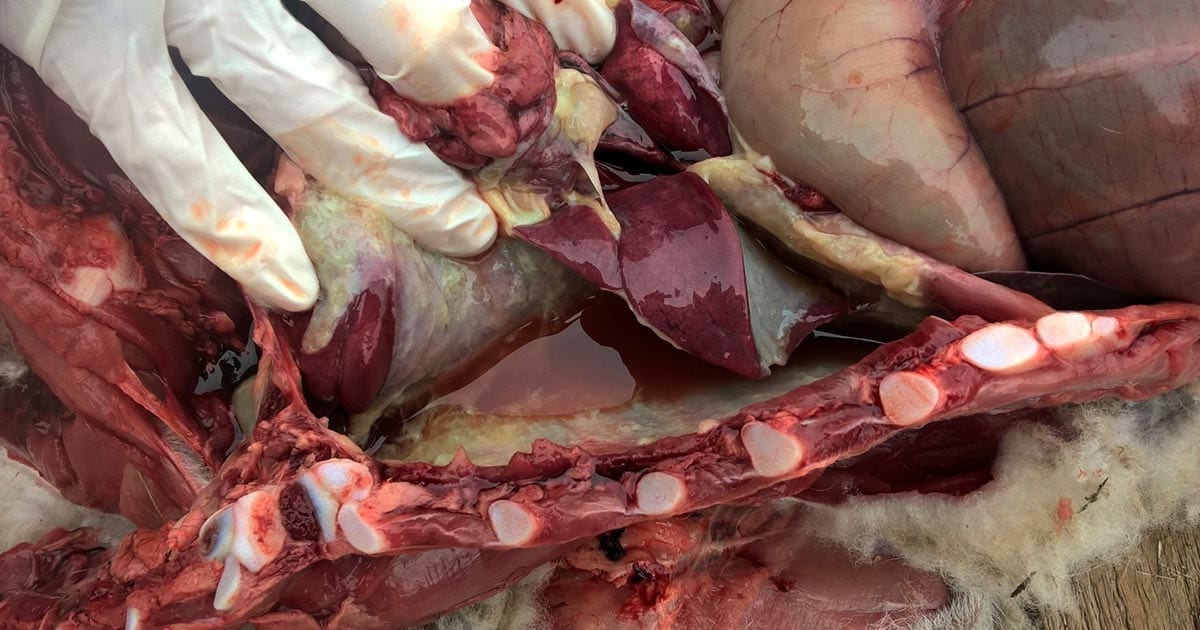
Figure 1. Septicaemic pasteurellosis.
Pasteurellosis is an infection with a species of the bacterial genus Pasteurella. The bacterium manifests itself most commonly as pneumonia, but also abortions, mastitis, bacteraemia and septicaemia.
Mannheimia haemolytica is a Gram-negative opportunistic pathogenic organism of the family Pasteurellaceae, and an obligate commensal of the upper respiratory tract of sheep and cattle. M haemolytica was previously named Pasteurella haemolytica, of which two biotypes existed – A and T. Thirteen A serovars and four T serovars existed. The T serovars were reclassified as Pasteurella trehalosi, now Bibersteinia trehalosi. After further reclassification, 12 M haemolytica serovars were identified – A1, A2, A5, A6, A7, A8, A9, A12, A13, A14, A16 and A17. M haemolyticaserovar A11 became Mannheimia glucosida.
serovar A1 and M haemolytica serovar A2 are the predominant serovars in cases of bovine and ovine pneumonia, respectively (Davies and Donachie, 1996). However, M haemolytica serovar A6 is increasingly prevalent (Zecchinon et al, 2005).
M haemolytica pneumonias occur in sheep, cattle and goats as the leukotoxin, the main virulence factor of M haemolytica, is cytotoxic only for ruminant leukocytes. Other virulence factors allow M haemolytica to evade host defences and proliferate in the lung – for example, adhesins that aid colonisation, and neuraminidase that reduces the viscosity of respiratory mucus and allows closer bacterial apposition to cell surfaces (Zecchinon et al, 2005).
Bacterial pneumonias due to >M haemolytica, often referred to as mannheimiosis, can be primary or secondary. M haemolytica is a commensal of the nasopharynx, and a degree of immunosuppression or disruption of the lungs’ protective mechanisms can result in pneumonia. Secondary infections with M haemolytica can often be preceded by infection of the respiratory epithelium, with viruses such as bovine respiratory syncytial virus, bovine herpesvirus type one, bovine coronavirus and bovine viral diarrhoea. Mixed infections with other bacterial agents – such as Mycoplasma bovis, Pasteurella multocida and Histophilus somni – are common (Griffin et al, 2010).
In ovine pasteurellosis, Mycoplasma species, ovine pulmonary adenocarcinoma, maedi visna and lungworm may precede M haemolytica infection (Bell, 2008a). Immunosuppression due to tick-borne fever or border disease may also contribute to a rise in cases in a flock. Clinical signs can vary from a nasal discharge and mild cough to sudden death. Acutely affected animals are often pyrexic and dyspnoeic.
Septicaemic pasteurellosis is caused by M haemolytica, and causes respiratory disease and sudden death in 6-week-old to 12-week-old lambs. Lambs appear tucked up, lethargic, pyrexic, dyspnoeic and, often, cyanotic. Lambs are often found dead. Postmortem examination shows fibrinous peritonitis, pericarditis and pleurisy (Figure 1; Sargison, 2008).
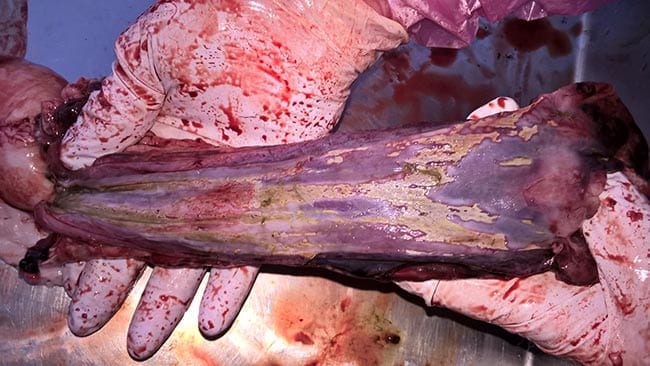
Systemic pasteurellosis due to B trehalosi affects lambs weaned in autumn and often presents as sudden death. Postmortem findings are indicative of general septicaemia, such as SC ecchymosis over the brisket and throat area. Characteristic lesions include mucosal necrosis in the pharynx, oesophagus and stomach (Figure 2; Lovatt et al, 2014).
Control of pasteurellosis and mannheimiosis in ruminants involves minimising stressful scenarios, where possible. This can be stress due to handling, housing, husbandry procedures, change of nutrition, mixing animals and changeable weather. Housing is a risk factor in cattle and sheep, and attention to ventilation, humidity and stocking density can reduce disease chances.
Ovine Pasteurella vaccines are available in the UK, usually combined with clostridial components. They protect against pneumonia and septicaemic pasteurellosis due to M haemolytica, and offer some protection against systemic pasteurellosis caused by B trehalosi (Sargison, 2008). Administering a booster vaccination to ewes four to six weeks pre-lambing allows lambs to acquire antibody protection from the colostrum, which is estimated to last for the first three to four weeks of life (Bell 2008b). It is advised a supplementary booster of vaccine is administered two weeks to three weeks before expected seasonal bouts of pneumonia.
Multiple injectable bovine vaccines containing inactivated M haemolytica serovar A1 are available in the UK. They require two doses as a primary course – this prohibits the protection of calves from a young age.
Makoschey et al (2012) demonstrated cows immunised with a vaccine based on iron-regulated proteins of M haemolytica conferred protection to calves via colostrum up to three weeks old. Although the experimentally infected calves displayed clinical disease, the survival rate was considerably higher in vaccinated calves.
serovars are sensitive to penicillin, ampicillin, oxytetracycline, erythromycin, streptomycin and amoxicillin/clavulanic acid (Scott, 2011). In sheep, oxytetracycline is often the treatment of choice, with a single, long-acting injection at 20mg/kg bodyweight (Bell, 2008b).
Tilmicosin is licensed in the UK to treat pneumonia in sheep associated with M haemolytica and P multocida. Prompt detection of affected sheep is required for treatment to be effective. Cases of antimicrobial resistance are most commonly found against beta-lactam antibiotics, tetracyclines, sulfonamides and aminoglycosides (Zecchinon et al, 2005). Due to the endotoxaemia and bacteraemia associated with pasteurellosis, NSAIDs are recommended in conjunction with antimicrobial therapy (Scott, 2011). However, no NSAIDs are licensed for use in sheep, so must be used under the cascade. NSAIDs are also advocated, alongside antimicrobials, in the treatment of bovine respiratory disease (BRD), with many licensed for use in the UK.
P multocida is classified into 5 serogroups – A, B, D, E and F – and 16 somatic serovars, and affects many species. The main serovar involved in BRD is P multocida serovar A3, which is more commonly seen in younger calves (Griffin et al, 2010). Angen et al (2009) tested transtracheal aspirated bronchoalveolar fluid from normal and clinically affected calves, by culture and molecular methods, and found P multocida was isolated from 48% of clinically unaffected calves.
Isolating P multocida from a nasal swab or bronchoalveolar lavage in a bovine with BRD is not necessarily significant. However, isolation from the lung tissue during postmortem examination is highly significant.
No commercial vaccines are available in the UK for P multocida, which can cause acute pneumonia, septicaemia and neonatal bacteraemia in sheep (Sargison, 2008). Control methods are similar to those for M haemolytica.
M haemolytica and M glucosida are known to be important causes of ovine mastitis, and more commonly found in meat flocks than dairy sheep production systems, where Staphylococcus aureus predominates (Bergonier et al, 2003). In a study of clinical mastitis in 70 lowland flocks in England and Wales, the most common M haemolytica isolates were M haemolytica serovar A2, followed by M glucosida and M haemolytica serovar A6; with 12.5% of the isolates not typable (Jones and Watkins, 1998).
Clinical signs include anorexia, tachypnoea, pyrexia, enlargement, hardening and erythema of the udder, and pain (Omaleki et al, 2011). Lambs suckling, teat injuries, poor nutrition, multiple progeny and multiparity are risk factors for Mannheimia mastitis.
M haemolytica, as a commensal of the nasopharynx, can be transferred to the teats via lambs sucking, but can also survive in the environment on bedding and pastures where sheep with pneumonia have grazed. It has been suggested controlling pneumonia in the adult flock will, therefore, decrease the transmission of M haemolytica to the ewe’s udder (Omaleki, et al 2011).
Reducing teat damage by early weaning and adequate nutrition will, subsequently, decrease the number of mastitis cases. Genetic selection of ewes with good udder conformation will maximise lamb sucking behaviour and minimise teat damage.
The efficacy of killed M haemolytica vaccines against Mannheimia mastitis has not been trialled. Barber et al (2006) carried out sensitivity testing on 13 isolates of M haemolytica – the isolates were susceptible to trimethoprim, tetracycline and ceftiofur. A benefit may also exist to administering NSAIDs alongside antimicrobials; as aforementioned, these must be used under the cascade.
Pasteurellosis affects sheep and cattle, and can vary in its clinical presentation. Immunity from vaccination is not absolute, and with the development of resistance to commonly used antimicrobials, attention needs to be focused on prevention and control.