5 Feb 2018

Infectious bovine rhinotracheitis is a relatively common disease, primarily in dairy cattle in the UK, caused by bovine herpesvirus-1. It can cause outbreaks of severe, mainly respiratory disease, but can also be associated with milder signs, including conjunctivitis and minor milk drop, as well as reproductive, digestive, nervous disease and possible productivity effects. Latent infection and recrudescence poses difficulties for diagnosis and control of disease within a herd. Options for control include biosecurity, vaccination and accreditation schemes.
Infectious bovine rhinotracheitis (IBR) is an important disease of cattle caused by bovine herpesvirus-1 (BHV1/BoHV1).
As a herpes virus, infection results in life-long infection of the host, with reactivation and shedding responsible for disease spread. A similar process results in shingles in adult humans following infection with the chicken pox virus as children. A positive antibody test indicates the animal is infected.
The virus has worldwide distribution and was first diagnosed in the 1950s in the US. Two main sub-types exist – BHV1.1 and BHV1.2 – with some differences in shedding in cattle between sub-types (Nettleton and Russell, 2017) and disease presentation.
BHV1 can cause IBR (upper respiratory tract disease and milk drop), but also uncommonly reproductive disease, known as infectious pustular vulvovaginitis (IPV) in females and infectious pustular balonoposthitis in males. It can also result in abortion, although this is not a common diagnosis in the UK.
Prevalence and disease incidence can vary, though in endemically affected herds usually at least half of the animals are seropositive.
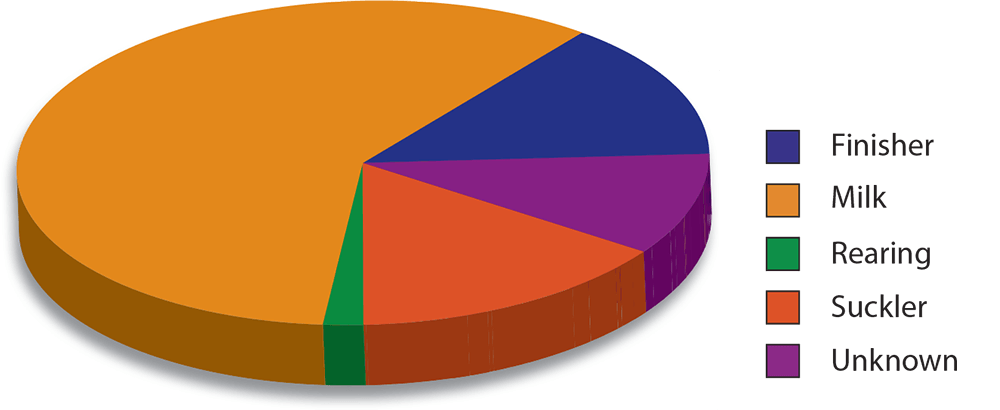
In 1998, antibodies to IBR were detected in the bulk milk of 69 per cent of dairy herds tested in England and Wales (Paton et al, 1998), and, in south-west England, Woodbine et al (2009) found antibodies in at least one animal in 83.2 per cent of herds. Generally, the disease has a higher prevalence in dairy herds (Nettleton and Russell, 2017; Figure 1). The disease has been eradicated in some European countries, including Austria, Denmark, Finland, Norway, Sweden and Switzerland.
The disease is most commonly introduced into a herd by purchased animals or by relatively close contact with infected cattle (the virus can be spread by aerosol up to 5m). Large quantities of virus are shed during primary clinical disease and from recrudescence of infection in latently infected animals.
In infected dairy herds, young cattle often remain naive until they join the milking cows, at which time clinical disease can occur. The virus can survive for up to a month at low temperatures/high humidity, and nasal excretions on clothing and other fomites can spread the disease to other farms (Nettleton and Russell, 2017). Semen from infected bulls can also spread the disease (Animal Health Ireland, 2016).
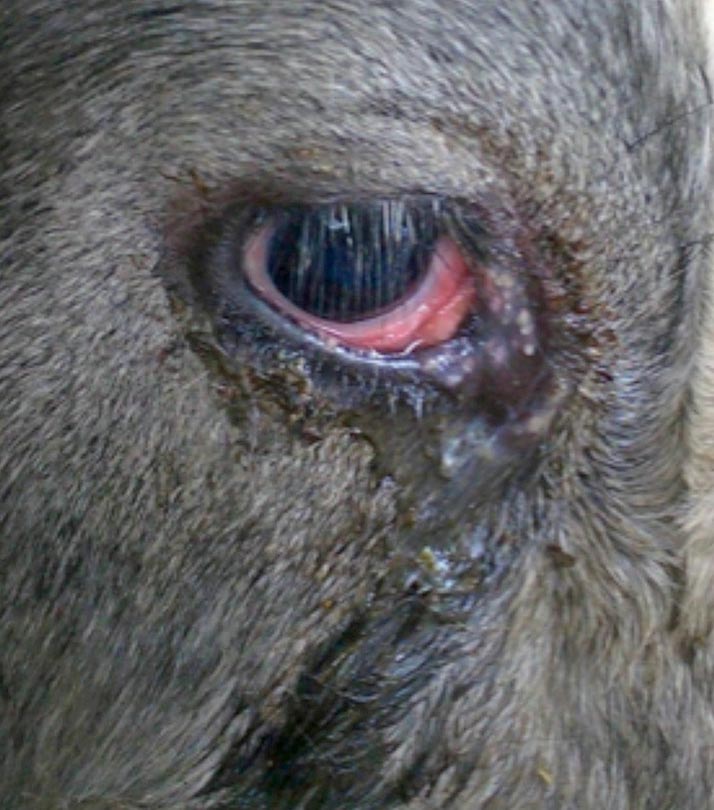
The four clinical presentations are respiratory, reproductive, digestive and nervous, with by far the most common being respiratory (upper respiratory tract) – often in heifers or newly purchased cattle.
Classic IBR is essentially a disease of the upper respiratory tract and can vary from mild signs, including:
In calves, IBR is not a common component of outbreaks of pneumonia, but severe disease has been described in neonatal animals (Nettleton and Russell, 2017).
In adult animals, milk drop with mild clinical signs is perhaps most common, but significant outbreaks of disease in naive herds can be seen (severe milk drop, deaths due to severe tracheitis and bronchopneumonia). Productivity effects, including reduced milk yields (Statham et al, 2015) and reduced fertility, have also been reported.
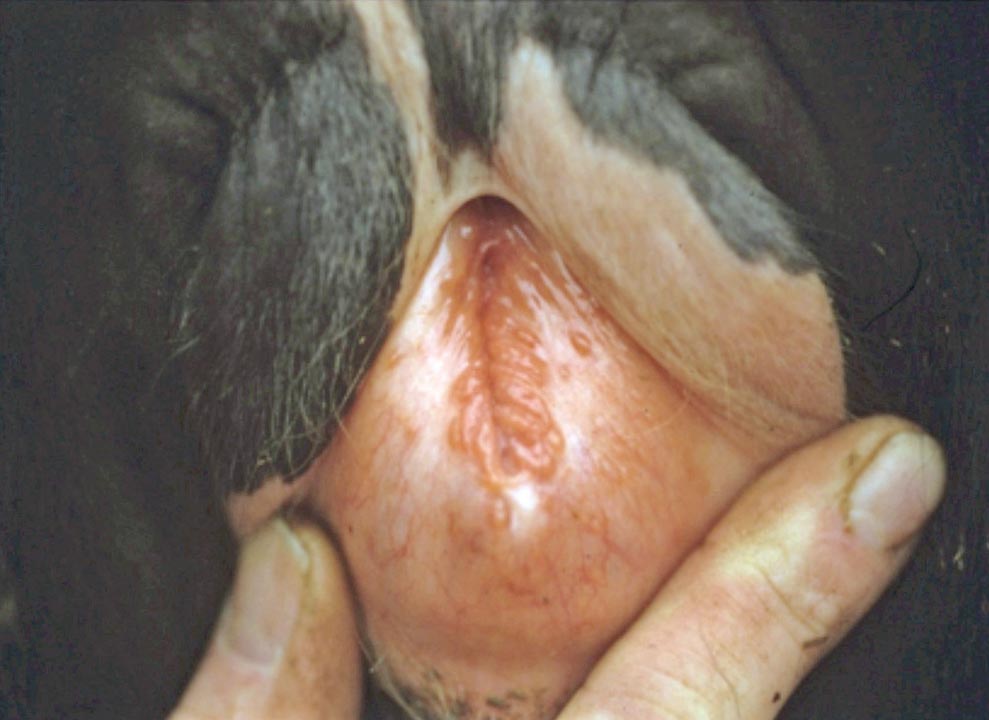
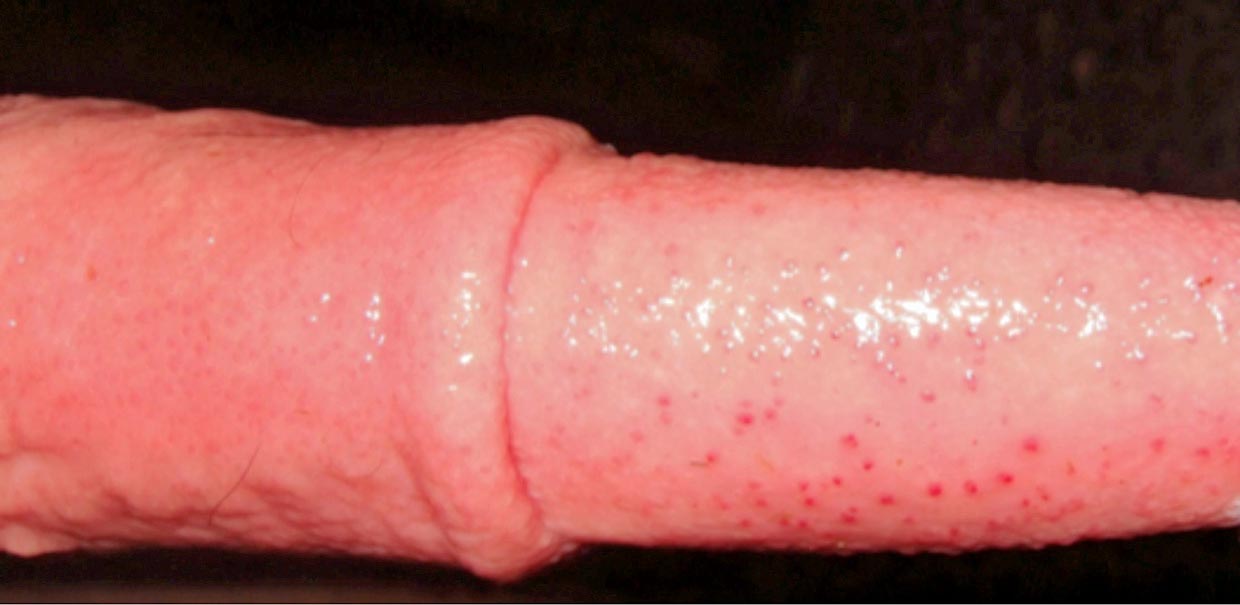
Digestive presentations are a very uncommon manifestation of IBR usually seen in calves, and may follow an outbreak of respiratory disease in older cattle. Necrotic ulcerative lesions may be seen in the upper alimentary tract (the oropharynx and oesophagus). Usually, respiratory signs are seen in these animals as well, but IBR could be considered if ulcerative lesions are seen in the gastrointestinal tract of calves at postmortem.
Nervous presentations are also an unusual manifestation of IBR infection and may be an extension of oropharyngeal infection in calves, and in older cattle may be blood-borne or from the nasal mucosa, resulting in encephalitis (Penny et al, 2002).
Serology (detection of antibodies in serum or milk) and virus detection (PCR) testing are the cornerstones of diagnosis. Specific requirements for sample collection can be found in the APHA guide on sample and test selection, but essentially:
Classical IBR associated with the death of an animal will almost certainly be associated with a severe rhinotracheitis (Figures 5 to 7) and secondary (aspiration/bacterial) pneumonia, often with pleurisy (Figure 8) due to bacteria, including Mannheimia haemolytica (Figure 9).
In these cases, a diagnosis is usually readily obtained by rubbing a plain swab on the mucosa of the trachea or bronchial bifurcation (Figure 10).
Postmortem blood can also be collected and will usually be suitable (if not severely haemolysed) for serology – bearing in mind sufficient time may not have passed for seroconversion. Tissue (trachea and lungs) can also be collected in formalin as histopathological changes can be diagnostic – immunohistochemistry is available and in situ virus can be demonstrated (Figure 11).
No specific treatment exists for IBR, only supportive treatment with antibiotics and NSAIDs. Isolation of animals showing clinical signs is recommended.
Control centres on ideally maintaining a free herd (closed herd or screening of purchased animals and biosecurity – infection can be introduced on fomites) and/or the use of vaccination to control infection within a herd, or aid eradication.
Various herd accreditation schemes are available and provide a structured approach to aid control and eradication.
Vaccination can prevent clinical disease and reduce virus spread, but does not prevent infection in animals exposed to virus (Nettleton and Russell, 2017). Marker vaccines are most commonly used in the UK (although non-marker vaccines are also still available).
Exposure to field virus can be differentiated from vaccinal antibody through the detection by a specific cELISA of gE glycoprotein, which is absent in marker vaccines – differentiating infected from vaccinated animals or DIVA technology. However, non-marker vaccines are commonly part of combination vaccines, which can complicate using serology to detect field virus exposure, particularly if the history of purchased animals is uncertain.
Live and inactivated vaccines are available:
Live vaccines are better at providing protection from clinical disease in vaccinated animals, but inactivated vaccines are better at limiting shedding in those infected animals.
One vaccination protocol that may provide good control of disease and aid in eradication on a herd level includes:
This combination is believed to reduce or prevent recrudescence of virus from carrier cows, thus help eradicate infection within a herd (Forrest, 2014).
IBR in cattle is caused by a herpes virus, so infected animals remain infected for life (vaccination does not rid an animal of infection) and serologically positive animals should not be purchased into an IBR free herd. It is primarily a disease of the upper respiratory tract, but severe disease can result in secondary pneumonia. It is not commonly part of the calf pneumonia complex, and less commonly can cause reproductive disease (including abortion and IPV/IPB), and digestive and nervous disease/pathology.
Common clinical signs include nasal and ocular discharge, and coughing and sneezing with or without pyrexia, and severe disease may be seen in naive animals. PCR is a sensitive test for diagnosis, and serological tests are also valuable aids to diagnosis, and can differentiate vaccinal antibody from field virus exposure (assuming marker vaccine is used).
Vaccine protocols are available that aid recovery after infection (live vaccine) and reduce virus shedding in infected animals (inactivated vaccines), and these may be part of both herd level and national level eradication schemes.