19 Oct 2015

Figure 4. Painful animals may be profoundly depressed with their eyes half-closed or shut, not grooming themselves, showing abdominal pressing and isolating themselves from other animals.
Improvements over the past 20 years in how we provide medical and surgical care for our exotic pets have resulted in healthier and longer lives for many of these animals.
Many owners are dedicated to providing excellent care for their pets, but the veterinarian also needs to be aware of their anatomical, physiological and behavioural peculiarities, so when a patient is presented with a health problem, he or she can provide appropriate and specific treatment.
Pain recognition and management is certainly one of the common challenges the clinician has to face in his or her daily practice. The ability to experience pain is universally shared by all mammalian species and rabbits are no exception. WSAVA has developed global guidelines on the recognition, assessment and management of pain in companion animals (www.wsava.org). This certainly represents a useful resource for those wanting to deepen their knowledge on this matter.
Pain has been defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage” (IASP, 2015). It is generally accepted “any procedure or disease likely to cause pain in human beings, should be expected to cause pain in an animal” (Kohn et al, 2007; Lichtenberger and Ko, 2007).
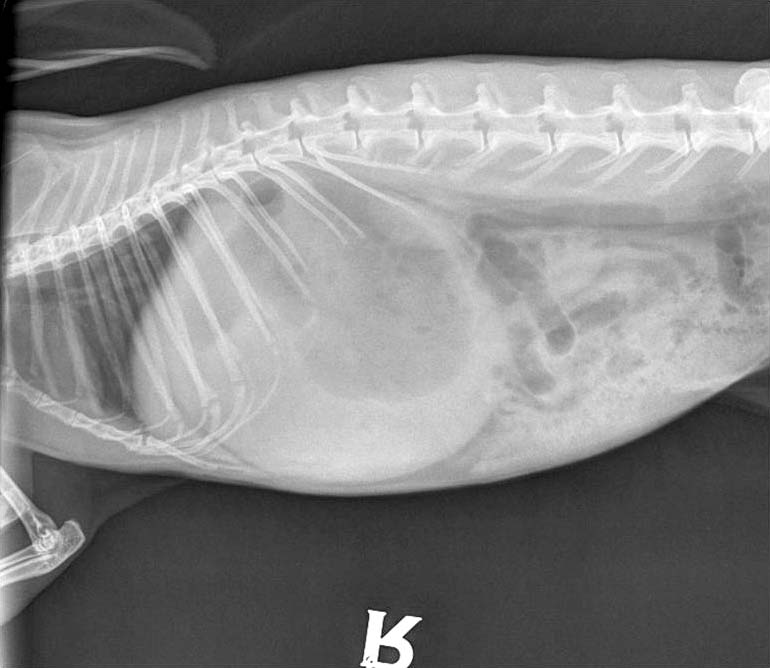
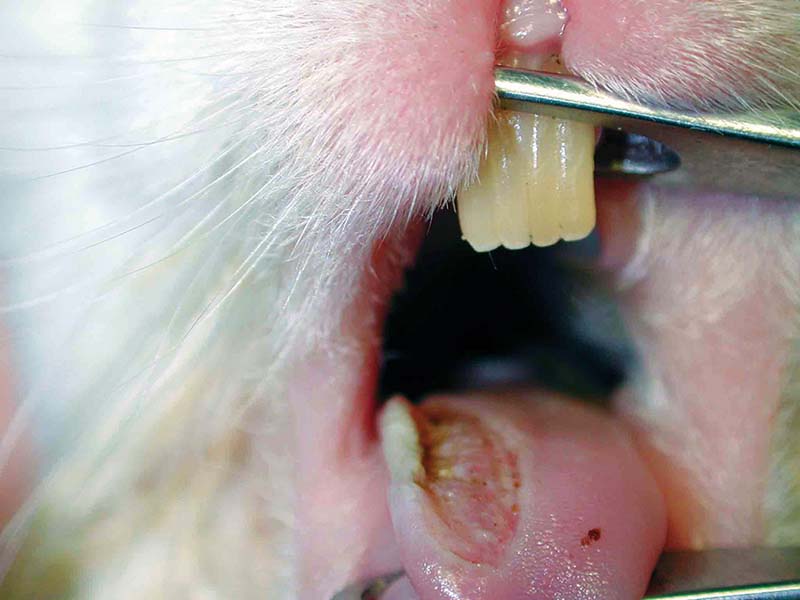
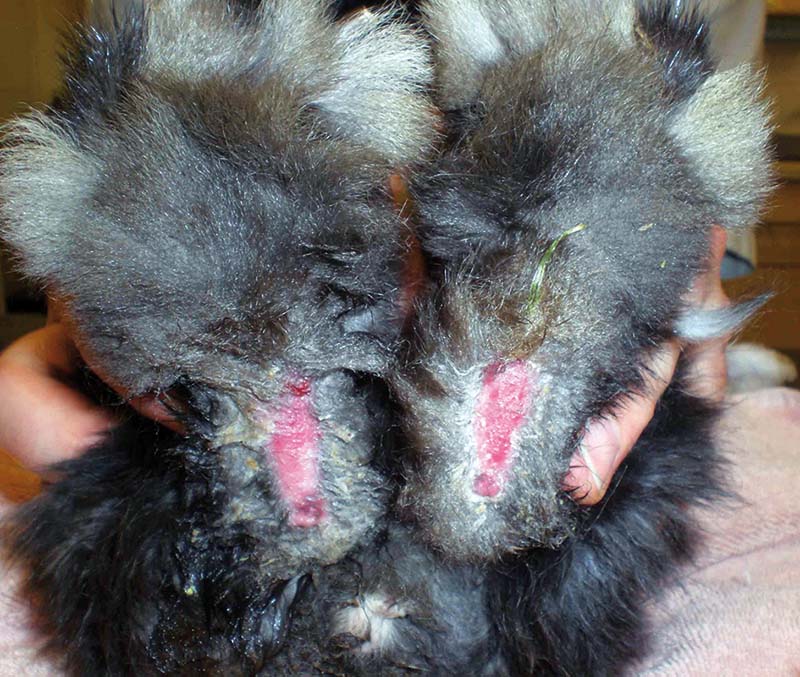
Pain recognition and control is therefore necessary – whether treating known painful conditions such as gastrointestinal stasis, dental disease, severe pododermatitis (Figures 1 to 3), trauma, burns or fractures, or simply when premedicating a rabbit prior to a procedure likely to cause pain.
The aim is to improve patient comfort, but also to avoid undesirable effects such as activation of the sympathetic nervous system, complement cascade, cytokine systems and arachidonic acid cascade (Barter, 2011), which may result in a variety of pathological outcomes including tachycardia, hypertension, reduced renal perfusion, hyperglycaemia, altered cardiac output, increased myocardial oxygen demand, altered physiological and endocrinological function, immune system suppression and even pathological lesions.
Other detrimental effects may include reduced appetite to anorexia, reduced gut motility eventually leading to altered gut function and stasis, and delayed wound healing and recovery times. All these issues may potentially be life-threatening to the rabbit (Varga, 2014). Rabbits are considered catecholamine-driven animals, very prone to stress and with a high metabolic demand. If a rabbit is already ill, this process can worsen dramatically its clinical conditions, resulting, in extreme cases, in cardiac failure and death due to stimulation of the sympathetic nervous system. Every attempt, therefore, must be made to reduce pain and stress, including provision of adequate pain relief at appropriate times (pre-emptive analgesia may help reduce pain and postoperative stress; Page et al, 1998).
To be able to alleviate pain, one must first be able to recognise it, to assess its intensity and identify its source and duration. Understanding the pain pathway (transduction, transmission and perception) allows us to chose the appropriate analgesic plan, targeting the pain pre-emptively (prior to a painful stimulus being applied, therefore potentially reducing changes in the nervous system in response to a noxious stimulus), intraoperatively and/or postoperatively with the awareness of the fact established pain is more difficult to alleviate and control (Barter, 2011).
It may be difficult to recognise animals in pain because they cannot express pain verbally, different species may have different behavioural responses to noxious stimuli, and because there may be individual differences in pain tolerance (Hawkins, 2006). Moreover, exhibiting normal behaviour is not necessarily an indication the animal is not in pain, therefore recognition of pain is often based on experience, familiarity with the different species and their normal behaviour, and the knowledge of likely causes of pain that may guide the clinician’s professional judgment (Kohn et al, 2007).
As prey species, rabbits are inclined to mask symptoms of disease – especially in an unusual environment or in the presence of an observer, who may induce immobility and freezing in the patient – with no apparent pain-related behaviour, making recognition of clinical signs even more difficult. However, acute traumatic or postoperative pain may also induce immobility in rabbits. Animals in pain may be profoundly depressed, with their eyes half-closed or shut, not grooming, avoiding attention and isolating themselves from other animals (Figure 4). Bruxism may be seen. An abnormal body position, such as a hunched posture and abdominal pressing, or change in temperament, have also been observed associated with pain (Hawkins, 2006).

Attempts have been made to validate pain scoring methods. Unfortunately, no objective, universally applicable method exists to assess and classify pain in animals. This remains a very complex process, often based on clinical impressions. Physiological parameters, such as heart and respiratory rate, blood pressure, plasma cortisol/corticosterone and/or catecholamine concentrations, have sometimes been used to assess pain in human and non-human species.
Many factors, though not pain-related, can influence these parameters (Livingston and Chambers, 2000). It has also been thought the same parameters used to indicate stress may be used to assess pain. Evaluation of behaviours and/or the return of normal behaviours and movement, respiratory rate, heart rate, bodyweight change, food and water consumption, faecal output, general appearance and demeanour are commonly used to evaluate pain in animals and often used postoperatively (with or without analgesia) to measure nociception and analgesia in various species including rabbits (Hawkins, 2006; Leach et al, 2009; Weaver et al, 2010).
An ethogram was developed for assessment of post-ovariohysterectomy pain in rabbits. In this paper, the authors concluded pain/stress associated with ovariohysterectomy induces changes in frequency and duration of a number of behaviours, and that assessement of these may be an effective way to identify an animal in pain (Leach et al, 2009). Observing and recording behaviours as a pain assessment method may be difficult without knowing exactly where to look and what to look for, as type and location of potentially painful procedures may vary.
According to a study, focusing only on the face was an ineffective behavioural assessment method to identify pain in New Zealand white rabbits as other body areas can also be involved (Leach et al, 2011). Nevertheless, pain-related facial expressions can be very useful and could improve our understanding of how rabbits feel and our ability to recognise when they are in pain. More recently, the rabbit grimace scale was developed as an accurate and reliable way of assessing acute pain in rabbits (Keating et al, 2012). In this study, facial expressions (such as orbital tightening, cheek flattening, nose pointing and whisker change) were used as behavioural markers of acute pain.
Pain can delay healing, lower immune responses and increase morbidity and mortality (Barter, 2011). Appropriate analgesia is therefore expected to allow a more rapid return to a normal behaviour in mammals (Hardie et al, 1997). An optimal pain management plan involves the use of multiple drugs acting at different levels of the pain pathway (multimodal analgesia), so smaller doses of each drug can be used – thereby enhancing pain relief and reducing the likelihood of side effects (Lichtenberger and Ko, 2007; Fisher, 2010).
The major classes of analgesic drugs used to manage acute and chronic pain in exotic animal practice include opioids, NSAIDs and local anaesthetics – for example, an opioid could be used to premedicate a rabbit undergoing surgery to modulate pain. A local anaesthetic block may be used to inhibit pain transmission and an NSAID could be administered perioperatively to alter pain transduction. Evidence also shows recovery can be hastened if opioids, NSAIDs and local anaesthetics are used to pre-emptively block the sensitisation of the CNS from a noxious stimulus before the tissue injury occurs (pre-emptive analgesia; Katz et al, 1992; Reichert et al, 2001). Selection of the appropriate analgesic protocol should include knowledge of pain physiology and the pharmacokinetic properties of that specific drug in a particular species (Kohn et al, 2007; Lichtenberger and Ko, 2007).
Opioids act centrally to limit the arrival of nociceptive information to the CNS and may also act peripherally in inflamed tissue (Barter, 2011). Their effects and dosages are variable depending on species, source, type of pain and route of administration (Lichtenberger and Lennox, 2009; Wenger, 2012). They are used for their potent and rapid onset of action in alleviating moderate to severe acute or chronic visceral pain (Flecknell, 1998; Kohn et al, 2007). They act by binding to μ or κ opioid receptors in the CNS or peripherally in inflamed tissues of mammals (Lichtenberger and Lennox, 2009). Rabbits, ferrets and other small mammals are considered sensitive to the respiratory and gastrointestinal side effects of opioids (Lichtenberger and Ko, 2007).
Used at the recommended doses, these drugs have a wide margin of safety and are very effective in alleviating pain. Their use is, therefore, highly desirable as the painful alternative can be even more deleterious (Lichtenberger and Lennox, 2009; Wenger, 2012).
Foley et al (2001) evaluated the efficacy of fentanyl patches in rabbits. Their study showed rabbits tolerate the transdermal patch well, although plasma levels achieved were variable and hair regrowth presented a complicating factor impeding dermal absorption of fentanyl after 24 hours.
NSAIDs act both centrally and peripherally to block nociception and decrease inflammation, thereby limiting the information directed to the CNS. They are generally used to prevent and alleviate acute postoperative and traumatic pain. Their anti-inflammatory, analgesic properties and their side effects are due to the inhibition of cyclooxygenase (COX) enzyme in the arachidonic acid pathway (Hawkins, 2006). The main concerns are related to the inhibition of prostaglandins synthesis, which may lead to side effects such as impaired renal function, gastrointestinal erosions and bleeding disorders (Lichtenberger and Lennox, 2009).
The main advantages from the use of these drugs are their prolonged duration of action and the fact they are not controlled drugs (Lichtenberger and Ko, 2007). Carprofen, ketoprofen and meloxicam are often used for effective pain relief in rabbits. Many authors recommend their use in stable, normovolaemic animals, post-surgery, when they start eating and are in presence of normal renal values, in view of possible intraoperative risks of bleeding and hypotension (Lichtenberger and Ko, 2007).
Meloxicam has certainly become one of the most commonly used NSAIDs in rabbit medicine. Because of its greater COX-2 selective activity it may be associated with decreased side effects (for example, inhibition of platelet function, gastrointestinal ulceration and renal function impairment (Jones, 2000). Several studies have been performed to evaluate efficacy, analgesic effects, safety and side effects of meloxicam administered to rabbits at different dosages and via various routes (Turner et al, 2006a and 2006b; Karachalios et al, 2007; Leach et al, 2009; Carpenter et al, 2009; Cooper et al, 2009; Fredholm et al, 2013; Goldschlager et al, 2013; Eshar and Weese, 2014; Delk et al, 2014; Khan and Rampal, 2014).
Rabbits seem able to metabolise meloxicam faster than dogs, humans and rats. It appears to be clinically safe in rabbits when used at appropriate dosages, but dosages as high as 1mg/kg may be necessary to achieve clinically effective concentration in this species. Significant alteration of the hard faecal microbiota did not appear to be a considerable adverse effect in rabbits treated for 21 days with oral meloxicam at a dose of 1mg/kg (Eshar and Weese, 2014). In one study, 1mg/kg followed by 0.5mg/kg was not sufficient to completely control pain after ovariohysterectomy (Leach et al, 2009). Therefore, multimodal analgesia should be considered for relief of pain associated with this surgery.
Local and regional anaesthetic techniques are widely accepted to provide good analgesia in small mammals (Hawkins, 2006; Wenger, 2012). Agents may be administered topically, locally (infiltration of the surgical site), through peripheral nerve blocks or epidurally to inhibit neural transmission to the CNS. Creams containing local anaesthetics (for example, a mixture of 2.5 per cent prilocaine and 2.5 per cent lidocaine) can be used to obtain topical anaesthesia prior to intravenous catheter placement or more invasive procedures (Wenger, 2012; Keating et al, 2012).
Lidocaine (1mg/kg to 2mg/kg in small mammals) and bupivacaine (1mg/kg) are the most common agents used, with bupivacaine prolonging the potency and duration of the local anaesthesia, but with higher toxic effects (Lichtenberger and Ko, 2007; Barter, 2011). The addition of morphine or buprenorphine to the mixture can potentially prolong the duration of the local anaesthesia by 10 and 9 hours respectively (Bazin, 1997).
Tramadol is an opiod-type drug whose analgesic effects result from a complex interaction between different receptor systems. It binds to μ-opioid receptors, and has some inhibiting activity on norepinephrine and serotonin uptake as well as α-2 adrenergic agonist activity. It has been described as inducing effective analgesia in humans with a lower incidence of adverse effects such as respiratory depression or constipation compared to other μ-opioid receptor agonists. Analgesic levels are not known in rabbits and oral administration of 11mg/kg only achieved variable plasma levels below those considered analgesic in humans (Souza et al, 2008). Data presented by Egger et al (2009) also suggested intravenous administration of tramadol to isoflurane-anaesthetised rabbits had some minimum alveolar concentration sparing effect. Higher doses may be clinically more relevant, as a reduced concentration of volatile anaesthetic used would be allowed, but may also result in a greater degree of cardiovascular depression.
Microdoses of α2-agonists, such as medetomidine or dexmedetomidine, can be used in combination with a tranquiliser or an opioid to provide analgesia, along with muscle relaxation and sedation. If used appropriately, these agents can minimally affect blood pressure and cardiovascular parameters (Lichtenberger and Ko, 2007).
Ketamine is often included in the anaesthetic protocol to reduce pain wind-up. Microdoses can also be given via the constant rate infusion route, in combination with an opioid, to provide some analgesic effect (Lichtenberger and Lennox, 2009).
Last, but not least, it is important to remind the veterinary surgeon of the benefits physical medicine can have if used alongside traditional analgesia. Cryotherapy, laser therapy, heat therapy, massage and rehabilitation can help in speeding recovery from trauma or surgery and have fewer adverse effects if used with awareness (Rychel et al, 2011; Ritzman, 2015). Alternative medical therapies such as acupuncture have also been increasingly considered to help reduce pain during the treatment of many common medical conditions. The neuromodulation acupuncture induces by direct nerve stimulation can alter pain transmission and aid in pain control and management (Koski, 2011).
Good pain management can also be achieved by providing a rabbit-friendly environment, appropriate housing and good husbandry practices in a stress-free environment with appropriate handling and adequate surgical techniques (Kohn et al, 2007; Varga, 2014).