26 Nov 2012

Figure 1. This obese rabbit is prone to GI statis due to immobility and poor diet.
Gastrointestinal (GI) stasis is a common and potentially life-threatening syndrome in rabbits.
A low-fibre diet, stress, or pain from an underlying illness are all major causes. In anorexic rabbits a full history and clinical exam should be performed. Hospitalisation is recommended to ensure prompt treatment and observation of food intake and faecal output. Other recommended diagnostic techniques are abdominal radiographs and blood sampling. Blood glucose gives an indication of pain and progression of the disease, helping the clinician decide if a case is medical or surgical. Low blood glucose is seen in rabbits with GI stasis, whereas obstructions likely to be surgical often have high blood glucose.
Treatment involves analgesia, prokinetics, fluid therapy and regular syringe feeding. Surfactants are often suggested, but lack of frothy bloat and inability to burp make their efficacy uncertain. Investigation and treatment of the cause must be carried out to prevent future episodes.
Treatment of complete obstructions is prompt surgical intervention. Obstructive problems can be prevented by avoiding locust bean pods and corn cob foodstuffs, and grooming to prevent large mats of fur. GI stasis is a multifactorial syndrome, but a routine lifestyle and a high-fibre diet are key preventive measures.
Gastrointestinal (GI) stasis in rabbits is an extremely common and potentially life-threatening syndrome.
The GI tract of the rabbit is designed for a near constant intake of low-quality, highfibre, low-carbohydrate diet. High fibre levels drive optimal gut motility, due to increased motilin production by the small intestine, and also provide the optimum environment for caecal microflora. A low-fibre diet can, therefore, result in gut hypomotility.
In GI stasis, normal peristaltic muscular contractions are slowed or, in some cases, cease. This results in decreased production of smaller and firmer faecal pellets, ultimately progressing to a halt.
Reduced GI motility reduces glucose absorption and the supply of nutrients and fluids to the caecal microflora. Alterations in caecal fermentation patterns can then lead to changes in caecal pH and volatile fatty acid (VFA) production. As the balance of caecal microflora changes, there is the possibility for proliferation of pathogenic bacteria, such as Clostridia species.
When stressed or in pain, rabbits release catecholamines that act to decrease gut motility; therefore, stress or pain from an underlying illness can be major causes of stasis. Unfortunately, rabbits are a prey species and signs of pain can be subtle (Panel 1). Other causes of stasis are obesity, chronic dehydration and adhesions. Trichobezoars (fur balls) are often implicated as a cause of gut stasis if found. It is more likely, however, that reduced gut motility leads to impaction and dehydration of stomach contents, resulting in trichobezoar formation.
When presented with an anorexic rabbit, a full history and clinical examination should be performed. Of particular importance are the following.
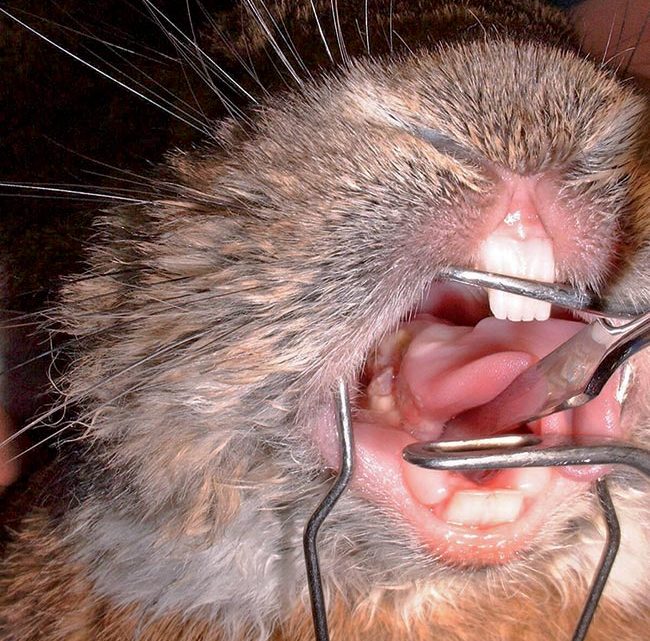
GI stasis and intestinal obstruction can be confused. Distinguishing between stasis and obstruction is important as each diagnosis requires different treatment. History and clinical exam provide clues as to which disease process is occurring (Panel 2).
Hospitalisation is recommended to ensure prompt treatment and observation of food intake and faecal output.
Gastrointestinal stasis:
Intestinal obstruction:
If taking abdominal radiographs fentanyl/fluanisone (Hypnorm, VetaPharma) provides good sedation, with the added advantage of analgesic cover and vasodilatory effects, which facilitate IV catheter placement (Longley et al, 2008). Radiographic changes in gut stasis include impacted material in the stomach and caecum and a possible halo of gas around them. Gaseous distension develops as stasis continues. In intestinal obstruction, however, fluid and gas may be seen proximal to the obstruction and bubbles of gas are seen in the stomach.
The packed cell volume of a hydrated pet rabbit is approximately 32 per cent to 40 per cent (Harcourt-Brown et al, 2001) – values of 45 per cent to 50 per cent can indicate dehydration. Blood glucose provides an indication of pain and progression (Table 1) and can be done simply and quickly with a glucometer. Individuals in stasis often present with lower blood glucose compared to the high values seen in obstruction. Evidence of lipaemia indicates hepatic lipidosis and is a poor prognostic indicator (Harcourt-Brown, 2002).
| Table 1. Interpretation of blood glucose levels in rabbits (Harcourt-Brown, 2011) | |
|---|---|
| Normal reference range 4.2mmol/L to 8.2mmol/L | |
| <2mmol/L | Significantly low – investigate. |
| 2mmol/L to 4.1mmol/L | No absorbing enough glucose/producing VFAs. Requires food and treatment for GI stasis. |
| 8.2mmol/L to 12mmol\L | Mild stress – for example, could be due to transport, trip to vets. |
| 12.1mmol/L to 15mmol/L | Stress – observe and resample. |
| 15.1mmol/L to 20mmol/L | Stress/pain – resample and investigate further to find cause. |
| 20.1mmol/L to 25mmol/L | Severe stress/pain. Requires prompt analgesia and may require surgery. Intestinal obstruction likely. |
| >25mmol/L | Critical – prompt, surgical intervention to find cause. |
Stretching of the gut wall by gas or food distension causes pain. Pain is a common cause of stasis and, even if it wasn’t present to start with, will compound the problem. Analgesia, therefore, is a must. Combining an opioid and an NSAID gives good multimodal analgesia. Buprenorphine and meloxicam are the authors’ drugs of choice (Table 2). The gut motility reducing effect of opioids seems insignificant in rabbits in normal use and is outweighed by the benefits of pain control.
Prokinetics help kick start the GI tract and are recommended in GI stasis. However, if intestinal obstruction is suspected, these should not be used.
Ranitidine is moderately palatable, easily available and fairly inexpensive. It also has antiulcerative qualities – useful in stressed rabbits that may also be on a high dose of NSAIDs. It is the authors’ first line choice.
Domperidone should not be given within two hours of ranitidine and not in conjunction with metoclopramide. It appears more effective than ranitidine alone. Thus, it is the authors’ second line treatment for recalcitrant or high-risk cases – usually with ranitidine.
Cisapride has re-emerged on to the veterinary market in tablet or suspension form, and appears to help some rabbits when ranitidine is not effective. Metoclopramide acts on the foregut and should, ideally, be used with ranitidine or cisapride for a synergistic effect. Cisapride and ranitidine may be used in conjunction, synergistically, at the lower end of their dose ranges.
| Table 2. Treatment protocols for rabbits in gastrointestinal stasis | ||
|---|---|---|
| Analgesia | ||
| Fentanyl/fluanisone | 0.15ml/kg to 0.3ml/kg IM, SC | |
| Buprenorphine | 0.02mg/kg to 0.05mg/kg q6-8 hours | |
| Meloxicam | 0.3mg/kg to 0.6mg/kg SC, PO q24 hours. Doses up to 1.5mg/kg are well tolerated for up to five days |
|
| Fluid therapy | ||
| IV Hartmann’s | 10ml/kg/hour to 20ml/kg/hour for the first two hours, followed by 100ml/kg/day |
|
| SC Hartmann’s | 10ml/kg to 20ml/kg divided into multiple sites | |
| Prokinetics | ||
| Metoclopramide | 0.5mg/kg to 1mg/kg SC, PO q6-12 hours | Acts primarily on the foregut |
| Ranitidine | 2mg/kg IV 3mg/kg to 5mg/kg PO q8-12 hours |
Acts throughout the gut. Also has anti-ulcerative effects |
| Cisapride | 0.5mg/kg (0.1mg/kg to 1mg/kg) q8-12 hours | Acts on the hindgut |
| Domperidone | 0.25mg/kg to 0.5mg/kg q12 hours | Acts on the foregut |
| Assisted feeding | ||
| Syringe feeding | 10ml/kg to 15ml/kg of liquid food three or four times daily | |
| Exercise | ||
| Lack of exercise is likely to further inhibit gut motility. Providing a large space to hop around in may help. When hospitalising rabbits, once or twice daily exercise is beneficial. | ||
| Abdominal massage | ||
| Gentle abdominal massage has also been suggested to promote passage of ingesta and relief of gas accumulation. It may help, but there are risks of gut perforation, especially if it is used in obstructive cases. Massage pads – commercially available for human back pain – have been suggested for rabbits to lie on. | ||
Fluid therapy is recommended. The aim is to rehydrate both the gut contents and the rabbit itself. Fluids can be given IV via the marginal ear vein. Topical anaesthetic sprays or creams can aid catheter placement in fractious rabbits. If unable to get IV access, fluids can be given subcutaneously, orally or via the intraosseous route.
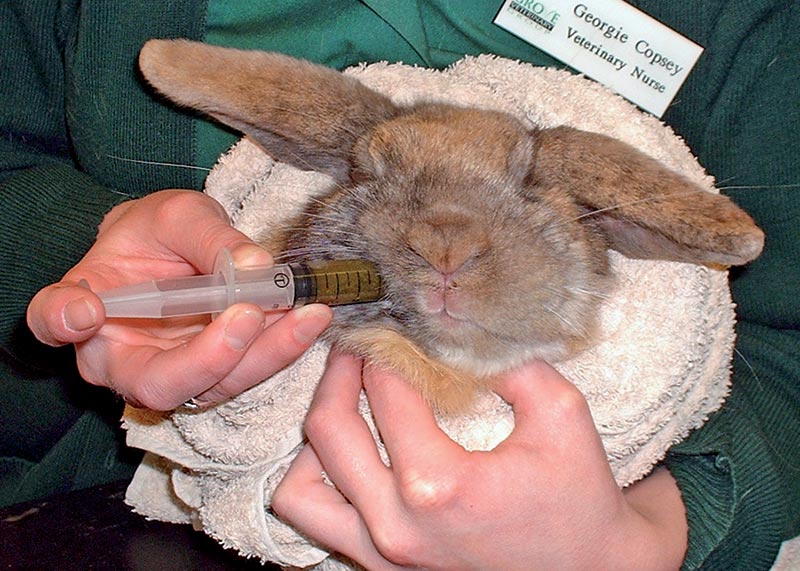
Assisted feeding is necessary in most cases of GI stasis (Figure 3). Anorexic rabbits start to utilise free fatty acids rather than glucose and VFAs as an energy source, leading to hepatic lipidosis and ketoacidosis. Hepatic lipidosis can develop within 24 hours of anorexia and the prognosis is to markedly worsen with duration.
In the early stages of anorexia, glucose alone may be sufficient, but as it progresses, protein, fat and fibre (to maintain gut motility and produce VFAs) are more important.
Surfactants, such as simethicone, are often suggested, but the lack of frothy bloat in rabbits and their inability to burp makes it uncertain how well this works.
In cases of GI stasis, investigation and treatment of the cause must be carried out to prevent future episodes. Prompt surgical intervention is required for complete obstructions.
The prevention of cases of gut stasis or obstruction is much easier than treatment. Obstructive problems can be prevented by avoiding access to foodstuffs, such as locust bean pods and corn cobs, or by grooming to prevent the build up of large mats of fur.
Non-obstructive stasis is a multifactorial syndrome, but a routine lifestyle with a stable hierarchy, changes only made very gradually and, most importantly, diet (Figure 4) are key factors in avoiding this unpleasant and potentially fatal situation.
