31 Oct 2022

Image © sarahdoow / Adobe Stock
“Hindgut disease” and “hindgut ulcers” are terms increasingly bandied about by horse owners with very limited understanding of their meaning.
Indeed, considerable confusion often exists about terminology; some horse owners even confuse pyloric lesions of glandular gastric disease with “hindgut” ulcers. This confusion is exploited by some supplement and feed companies to create a market for a range of products designed to “fix” hindgut disease and ulceration.
The intestines of the horse – especially the large intestine – are host to many billions of microorganisms responsible for digestion of structural carbohydrates in plants. Dysbiosis, a disruption to the intestinal microbiome, lies at the heart of most “hindgut disorders” in the horse (Figure 1). Recognition of dysbiosis is obvious in the case of diarrhoea, where pathogenic or opportunistic bacteria proliferate, resulting in colitis.
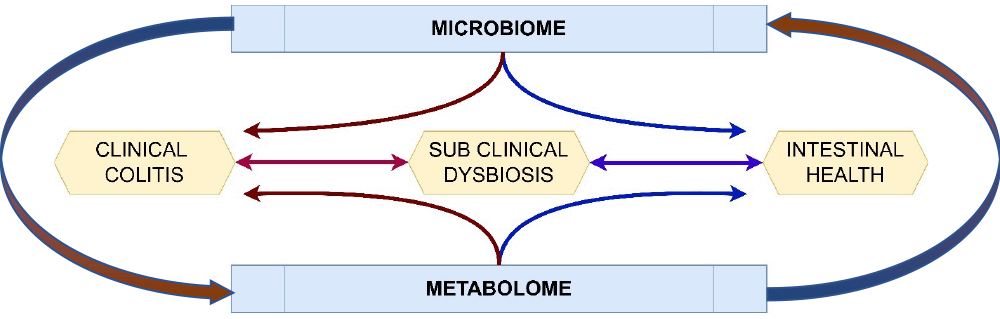
However, subclinical forms of dysbiosis undoubtedly exist in the horse that have been attributed with a wide variety of clinical presentations and potentially overlooked in clinical practice.
Moreover, the microbiome itself may not be the only contributor to intestinal health, with its metabolic products, such as volatile organic compounds, collectively referred to as the metabolome, playing an important role, potentially leading directly to inflammation of the wall of the colon.
Colitis presents as diarrhoea, with or without weight loss, that may be accompanied by hypovolaemia and hypoalbuminaemia. It can be caused by dysbiosis, parasites and a range of toxins.
Antibiotic-induced diarrhoea is a severe form of dysbiosis, where the microbiome is rapidly altered, allowing the proliferation of existing bacteria within the hindgut or colonisation by potentially contagious organisms.
Diagnosis can sometimes be confirmed by identifying specific pathogens (for example, Salmonella species, rotavirus, equine coronavirus or parasites), or their toxins (Clostridium species toxins).
Despite our best efforts, we often fail to isolate a specific pathogen and an assumption of intestinal dysbiosis is made based on the response to general nursing care.
Medicines and toxins can cause colitis, in particular the NSAIDs, which can cause right dorsal colitis (RDC), leading to severe protein-losing enteropathy.
A diagnosis can usually be made based on history of medicine administration and the ultrasound appearance of thickening of the right dorsal colon.
Toxins, such as the gallotannins of acorns and the therapeutic use of quinidine, can also cause specific inflammation within the large intestine. In certain geographical areas, sand accumulation in the ventral colon can be an important cause of colitis.
Subclinical dysbiosis (SCD) reflects a change in the intestinal microbiome without causing the obvious clinical sign of diarrhoea. It is a poorly understood phenomenon, but a growing body of evidence argues that changes in the microbiome are associated with a wide range of conditions (Table 1).
| Table 1. Example of the disorders that have been attributed to intestinal dysbiosis in the horse with a summary of evidence. Readers can access these articles using the DOI label (doi.org/number) in any web browser | ||
|---|---|---|
| Condition | Summary of current knowledge | DOI of reference source |
| Clinical conditions/states that have been associated with dysbiosis | ||
| Laminitis | Multiple studies indicating differences in microbiome between normal and horses with chronic laminitis. | 10.1186/1746-6148-8-231 |
| Obesity | Differences between lean and obese horses identified, as well as differences in metobolome. | 10.3389/fvets.2018.00225 10.1371/journal.pone.0215918 |
| Colic | Firmicutes and proteobacteria increased in postpartum mares with colic. Multiple other studies indicate “altered microbiome” in horses with colic with reduced diversity. |
10.1111/evj.1236 10.5455/OVJ.2022.v12.i2.12 |
| Headshaking | Methanocorpusculum increased. | 10.1002/vms3.735 |
| Colitis | Firmicutes, bacteroidetes and proteobacteria increased in colitis. | 10.1371/journal.pone.0041484 |
| Antibiotic associated diarrhoea | Antibiotic use results in changes in microbiome with limited differences in those that develop diarrhoea. | 10.3390/ani11061807 |
| Medications | NSAIDs and use of anthelmintics are associated with changes in microbiome. | 10.1016/j.jevs.2020.102943 |
| Physiological states | Age and stressors (transport, fasting or anaesthesia) are all associated with changes in microbiome | 10.1016/j.jevs.2020.102943 |
Some debate remains about whether the change in microbiome is truly the cause, or whether both the presenting complaint and the change in microbiome are the consequence of other factors. Unfortunately, diagnostic testing for SCD is unreliable and the wide variety of clinical signs that have been described for the condition are non-specific, often with significant overlap with equine squamous gastric disease, equine glandular gastric disease, as well as primary behavioural disorders (Panel 1).
Indeed, the clinical signs that have been described are so vague as to convince many owners that their horses suffer from hindgut disease.
To understand the significance of SCD, a brief understanding of the microbiome is relevant. The intestinal microbiome represents the populations of microbiomes throughout the intestinal tract, including stomach, small intestine and large intestine. However, most studies evaluate the large intestine, with the majority focusing on the faecal microbiome. It is important to recognise that these are not the same and populations vary throughout the intestinal tract in the normal horse.
The most recent studies identify bacterial populations using sequencing techniques based on ribosomal RNA (ribonucleic acid). These overcome many of the limitations of culture-dependent techniques that were previously used, but only identify the relative presence of each bacterial species, rather than their biological functions.
More recently, microbiome studies have been combined with metabolomic techniques that also document the biological functions of these organisms by measuring their endpoints of metabolism; for example, lactate, propionate and acetate.
The microbiome is responsible for liberating carbohydrates from plant materials. These carbohydrates are present in different forms: the structural carbohydrates (cellulose and hemicellulose), which have no direct nutritional value to mammals, and the non-structural carbohydrates (NSCs), the simple sugars, fructans and starch.
Structural carbohydrates are broken down by bacteria, largely in the large colon, while NSCs may also need processing to be utilised for energy. The simple sugars (disaccharides and polysaccharides) are rapidly processed and absorbed as glucose in the small intestine. Starch, a polysaccharide of glucose, is broken down by amylase into glucose and is absorbed in the small intestine.
However, horses have a relative deficiency of intestinal amylase production and, therefore, have limited small intestinal processing function of this sugar. These NSCs do not normally transit through the small intestine, unless consumed in excess (for example, grain overload), or if significant pathology is preventing its absorption (Figure 2).

When they do reach the large intestine, they provide readily fermentable energy sources for the microbiome, resulting in rapid fermentation and changes to the metabolome, often with an increase in lactate production and acidosis, that then favours changes in the microflora (SCD) due to changes in pH. Lumenal acidosis can result in intestinal inflammation, while rapid fermentation may also result in the liberation of gases (carbon dioxide and hydrogen, which are converted into methane) that may lead to tympany – potentially causing colic.
Fructans, complex polymers of fructose, cannot be hydrolysed by mammals, and must be broken down into fructose molecules by the small and large intestinal microbiome.
Diets high in fructan, such as some grasses, result in liberation of large amounts of fructose in the large colon due to the action of Streptococci species bacteria, again resulting in intestinal acidosis. The effects of fructans are complex; indeed, oversupply of oligofructoses has been used as a model for laminitis induction in the horse by their rapid bacteria breakdown by hind gut bacteria., and are likely involved in insulin dysregulation in naturally occurring laminitis.
However, breakdown by other bacterial species (for example, Bacillus species) has not been associated with similar effects, suggesting complex kinetics of the production of fructose from fructans by different bacteria.
Anecdotally, the addition of dietary fructanases in nutritional supplements, such as enzyme-rich malt extract, has improved insulin sensitivity in small numbers of horses. However, further evaluation of its impact in obesity and EMS is required before any recommendations can be made.
Horses have evolved to feed on high fibre (low NSC) diets. These favour microbial diversity, promoting breakdown of complex carbohydrates and releasing volatile organic compounds that amount to 50% of the energy provision to the horse.
These organisms also release factors that prevent the growth of commensal, potentially pathogenic organisms over those promoting intestinal health.
Appropriate nutrition is at the cornerstone of promoting intestinal microbial health. High carbohydrate content – especially high NSC content – should be avoided and starch should be limited in the normal horse to 1g/kg to 2g/kg daily.
In horses suspected of having SCD, benefit likely exists from further reducing this starch content. In the pasture-fed horse, reducing fructans is more complex and pasture restriction may also be required.
In the high-performing athlete, where starch feeding may be thought desirable by trainers, the addition of pre-digestive enzymic products may enhance small intestinal breakdown of fructans and starch.
In one study, a beneficial impact of an enzyme-rich malt extract supplement was shown upon the microbiome and metabolome in high-starch fed racehorses.
These products may offer a novel approach to the management of horses with SCD.
No reliable non-invasive diagnostic tests exist to confirm the presence of SCD, making research into therapeutic options extremely challenging. Documenting the microbiome is now possible using advanced molecular techniques that are not dependent on culture techniques.
These tools are often marketed direct to horse owners, with practitioners expected to interpret and act on those results.
The techniques are robust in terms of being able to identify the microbial population, but a number of limitations exist of even the most advanced laboratory techniques.
In short, while the technique is feasible, it is unknown if any value exists in trying to “normalise” the faecal microbiome.
While the intestinal metabolome, the metabolic outputs of microbial fermentation, is of considerable interest, no current commercial tests exist to aid in the evaluation of SCD.
As a proxy, faecal pH is sometimes used as a marker of colonic lactate production; however, it is a crude tool that suffers from many of the limitations of analysis of the faecal microbiome. It may have some value in assessing trends and impacts of different interventions in individual horses.
In horses with colitis, management largely relies on general supportive care and the use of IV fluid therapy.
Intestinal protectants and anti-inflammatory medications may be useful in some cases – in particular, the use of corticosteroids in the face of larval cyathostominosis is widely recommended.
The use of corticosteroids in other forms of colitis is more controversial, but likely has a place in a number of these cases; although, this should be considered in a risk-based approach.
Their use in SCD requires further study and at this stage, limited evidence recommends their use without some primary indicator of inflammation, such as ultrasound appearance of large intestinal inflammation.
No evidence exists as to the value of sucralfate, or indeed any intestinal protectant, in reducing caecal or colonic inflammation, and given the huge volume of the horse’s colon, it seems unlikely that the current recommended doses would have any impact on the large intestine in the horse.
Misoprostol is a prostaglandin analogue that is often recommended in the management of RDC to promote intestinal blood flow.
In horses where RDC has been induced by NSAID administration, NSAIDs should be discontinued, and alternative analgesic sources considered. While paracetamol is often considered appropriate in such cases, its analgesic effects in RDC without traditional concurrent NSAIDs is questionable.
Diet remains at the forefront of managing horses with dysbiosis, whether that is colitis or SCD. Reducing filling of the colon by using small, short fibre meals, comprising of alfalfa and or sugar beet, may reduce the workload of the microbiome, and improve the metabolomic profile in the large intestine.
The use of psyllium is also recommended as a valuable source of dietary fibre that promotes the production of short-chain fatty acids that may reduce intestinal inflammation.
Dietary oils may also be a valuable source of energy to the horse, with colitis not requiring fermentation and may have some anti-inflammatory effects.
Directly modifying the microbiome is often considered valuable in improving hindgut health and is widely recommended in horses with colitis. It is also increasingly being recommended for horses with SCD.
Probiotics, using lyophilised bacteria, have been widely reviewed for their value in modifying the microbiome; however, data of efficacy is lacking and in some studies, no viable bacterial species could be identified from commercial probiotics. Prebiotics aimed at providing the substrates to promote proliferation of some groups of microbes are also available and some impact on the microbiome has been identified; however, their use in SCD or colitis has not been evaluated.
Pre-digestive enzyme supplementation is a relatively novel avenue of research and has been evaluated in the horse, as well as a number of farmed animal species, including cattle and poultry, where it promoted energy liberation from foods.
Enzyme-rich malt extract has been shown to impact on the microbiome and metabolome in normal grain-fed racehorses, but an understanding of its value in clinical disease is lacking.
Given the horse’s relative lack of amylase and common access to fructans in grass, it is a species that could potentially greatly benefit from pre-caecal digestion and absorption, both in terms of improving energy efficiency and reducing the impact of carbohydrates on the large colon microbiome.
Faecal microbiome transplantation (FMT) is a re-emerging tool in the equine veterinarian’s toolkit, and becoming an exciting area of intervention for humans with Clostridium difficile diarrhoea and perhaps inflammatory bowel disease. Its use in SCD in human patients gives us a greater understanding of the complex interactions between the microbiome and its host.
While no specific guidelines exist, practical recommendations for how to undertake FMT in the horse have been published. To the author’s knowledge, FMT has not been applied to animals with SCD.
In summary, our understanding of dysbiosis in the horse is at a very early stage, and while equine clinicians apply their understanding of this to horses with colitis, opportunities to manipulate the microbiome in a wider range of disorders associated with SCD will help to demonstrate the importance of changes in the microbiome.
Reducing the proportion of NSCs and fructans entering the hindgut remains the most valuable technique in maintaining a normal microbiome and metabolomic profile in the equine large intestine. FMT and dietary supplementation are exciting opportunities for evidence-based research to improve intestinal health in the horse.