25 Feb 2025

Image: © michelangeloop / Adobe Stock
Over the past 25 years, we have increasingly recognised the major importance of hyperinsulinaemia in causing both subclinical laminar damage and clinical laminitis in equids (Patterson-Kane et al, 2018).
This has been referred to synonymously as “endocrinopathic laminitis” and, more lately, as “hyperinsulinaemia-associated laminitis” (HAL; Equine Endocrinology Group 2022). This is usually associated with three conditions: equine metabolic syndrome (EMS), pituitary pars intermedia dysfunction (PPID, equine Cushing’s disease) and therapeutic glucocorticoid administration.
It is worth noting these three conditions represent the sole cause of laminitis or may arise in combination, where the coexistence of two or even three of these may further increase the likelihood of laminitis. Occasionally, we see other causes of laminitis, such as following excessive weight bearing (for example, contralateral fracture) or systemic toxaemia (for example, colitis or metritis), but undoubtedly the vast majority of laminitis cases seen in practice will have been caused by excessive postprandial hyperinsulinaemia.
It is very important to remember, though, that acute clinical laminitis is only the tip of the iceberg. It is evident many animals suffer from chronic, insidious laminar damage, often without significant pain, but still eventually resulting in the same end point of anatomic failure of the laminar attachment (Figure 1).

Strictly, the diagnosis of hyperinsulinaemia-associated laminitis can only be made in the unlikely event that a blood sample had been collected and analysed shortly prior to the onset of laminitis. Collecting samples for basal insulin concentrations following an attack of laminitis can be misleading, as usually the animal will have had major management changes that will have impacted on insulin concentrations. For example, consider a pony that develops laminitis while grazing. By the time the veterinary surgeon attends a few hours later, the pony will usually have been stabled and eating nothing, or simply hay, resulting in a very different magnitude of insulin secretion than when the laminitis occurred. The general rule applies of “high insulin values being concerning, but low insulin values not being reassuring”.
Clearly, a dynamic test could be used here, such as measuring insulin following light corn syrup administration, to assess the presence of insulin dysregulation, although the risk-benefit balance of this is questionable given the extremely high likelihood of hyperinsulinaemia-associated laminitis in the absence of alternative overt causes (such as retained placenta or contralateral fracture).
Thus, it is reasonable to assume causation being hyperinsulinaemia unless evidence to suggest otherwise is obvious. Nevertheless, measuring insulin on the first visit to an acute laminitic remains highly valuable, not for diagnosis of hyperinsulinaemia-associated laminitis (which we might generally justifiably assume), but for assessment of the suitability of the ration that the animal is currently consuming (while now presumably stabled). Thus, a high resting insulin at this time indicates further changes in the diet are required immediately, whereas a low resting insulin suggests the current dietary intake to no longer be causing further laminar insult.
Acute laminitis is unlikely to improve if the horse is still experiencing episodes of hyperinsulinaemia and it is a crucial part of early laminitis management to ensure insulin remains low. We should never assume that hay is a safe feed in this respect.
Many hays are remarkably hyperinsulinaemic in individual horses, even when soaked. Remember that hay is dried grass. When the grass dries in the sun water evaporates, not sugar. Although soaking is an effective means of reducing the sugar content of hay, this happens to a very variable degree and the resulting sugar content of soaked hay will also clearly depend on how high it was pre-soaking, meaning that many soaked hays remain potentially dangerous.
At the time of the first visit to an acute laminitis case, one might also consider the use of a sodium-glucose cotransporter 2 (SGLT2) inhibitor drug while insulin status is being established (Meier et al 2018; Lindase et al 2023). SGLT2 inhibitors are remarkably effective at rapidly controlling excessive hyperinsulinaemia (if present) and could then be discontinued if the laboratory indicates acceptable insulin concentrations or further dietary modifications successfully control hyperinsulinaemia. There do not currently appear to be major short-term risks or disadvantages in the use of SGLT2 inhibitors in horses other than hypertriglyceridaemia, which can be checked after a few days of treatment.
Clearly, standard acute laminitis management should also cover analgesia, confinement and suitable foot support while the laminae regain cohesive strength, but the importance of constant maintenance of normo-insulinaemia in this period cannot be overstated.
A strong focus on control of post-prandial hyperinsulinaemia remains the centre point of long-term prevention of laminitis. In the past we may have focused too much on indirect causes of hyperinsulinaemia, such as obesity and lack of fitness. These two factors are clearly deserving of control via loss of fat mass and increased exercise, but generally prove hard to achieve due to weight loss resistance and also owner resistance to dietary restriction.
Additionally, given that a tendency towards excessive postprandial hyperinsulinaemia appears to have genetic influences, it is not uncommon to see cases where both weight loss and fitness have been achieved successfully, but the animal remains hyperinsulinaemic following sugar challenges – presumably due to strong genetic determinants in that individual.
An animal may be obese or lean, fit or unfit, PPID or non-PPID, EMS or non-EMS, a Shetland pony or a Thoroughbred, but in all cases the cause of hyperinsulinaemia is the same: recent dietary intake. The concept of “diet-resistant hyperinsulinaemia cases” is an oxymoron and should be discarded. If an animal (of any species) does not eat, it does not secrete insulin.
Experience indicates that in all cases it is possible to find a quality of diet that is not excessively hyperinsulinaemic, although admittedly it might prove difficult or impractical in some cases. Nevertheless, we should remember the principle that dietary modification is paramount in controlling excessive hyperinsulinaemia. This does not necessarily mean dietary restriction (which is more directed to fat loss), but is better achieved by finding an appropriate quality of diet. Often hay is fine, but, as already mentioned, it is not unusual to see dramatic hyperinsulinaemia after eating hay, even when soaked. This can only be determined by postprandial testing – for example, measuring insulin around two hours after the horse is allowed 0.4kg/100kg bodyweight of its own hay.
Should forage be found to be significantly hyperinsulinaemic (for example, insulin greater than 75mIU/L), then the test can be repeated after soaking the hay (where soaked hay is being checked then weigh dry prior to soaking), an alternative hay can be checked or straw can be mixed with the hay. Mixing straw with hay can be very effective in reducing insulin responses to forage while not significantly limiting forage access. However, this should be gradually introduced over no less than two to three weeks. Native ponies will generally tolerate up to 50% of the diet as straw, although greater care should be taken in other breeds, such as Warmbloods, to avoid the risk of colon impaction. In the early period, dentition should be checked, droppings output should be monitored and water intake encouraged.
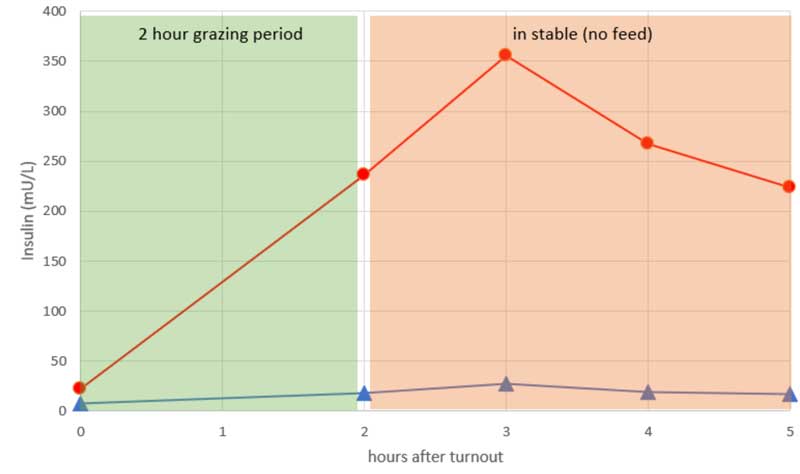
Similar postprandial checks can be performed following test grazing periods of no more than one to two hours (Figure 2). Insulin is generally best measured around an hour after coming off the grass. Initially a cautious approach should be used by testing a low-risk grazing period (for example, dull, warm day, early morning), although if all seems well then greater reassurance is found in low insulin values later in the day on “high-risk” grazing periods, such as cool sunny days where grass sugar content is expected to be higher. Inevitably, “grazing checks” are only valuable if repeated under different seasonal conditions.
While standing by the principle that dietary modification can control excessive hyperinsulinaemia in all cases, we do encounter cases where this proves impractical and the only available ration remains concerningly hyperinsulinaemic. This situation generally warrants consideration of SGLT2 inhibitor use in a more chronic fashion, while bearing in mind that these drugs remain unlicensed in equids and possible long-term adverse effects are poorly understood.
Nevertheless, given the alternative likely consequence of recurrent laminitis, most owners appear comfortable with offering informed consent for use of these drugs. Our current understanding is the benefits of these drugs are primarily to decrease the postprandial insulin response in a relatively acute fashion. Thus, the common question of “how long does the treatment course need to be?” is simply answered by “for as long as the diet remains inappropriate”. Thus, referring to the aforementioned section on approach to the acute clinical laminitis case, if the diet is changed to an appropriate ration today, the SGLT2 inhibitor can be discontinued tomorrow. On the other hand, if diet remains hyperinsulinaemic for the next 10 years then the drug will be required for the same duration.
Finally, a further important consideration when interpreting serum insulin concentrations is the dynamic nature of serum insulin. Consider a pony with a basal insulin of 268mIU/L on 17 March and then a further sample indicates 24mIU/L on 26 April. Does this indicate an improved status? Maybe. Or maybe not. Serum insulin concentration should never be regarded as a relatively static parameter like we might regard serum Gamma-glutamyl transferase or serum creatinine. It is highly dynamic and it is perfectly feasible to see two samples on the same day, taken only a few hours apart, differing by a similar amount, simply depending on their timing relative to whatever the animal ate that day. Thus, serum insulin can only ever be interpreted meaningfully in the context of dietary intake over the previous few hours.
Remember – the vast majority of laminitis cases are caused by hyperinsulinaemia. Only one cause of hyperinsulinaemia exists – recent dietary intake. All other factors, such as obesity, insulin dysregulation, EMS or PPID, will influence insulin concentrations, but are not a cause. Finding a balanced ration that is not excessively hyperinsulinaemic is the primary aim in managing all cases, without necessarily introducing harsh dietary restriction.