25 Mar 2025

Image © vprotastchik/ Adobe Stock.
Equine pituitary pars intermedia dysfunction (PPID) is a common, slowly progressive neurodegenerative disease associated with loss of dopaminergic (inhibitory) input to the melanotropes of the pituitary pars intermedia.
The pathophysiology of PPID is incompletely understood.
The loss of the hypothalamic dopaminergic inhibitory input appears to be associated with localised oxidative stress and abnormal protein (a-synuclein) accumulation, both of which lead to cytotoxicity and subsequent neuronal degeneration.
However, the exact cause of these changes remains unknown.
The consequent continual stimulation of this region results in hyperplasia of this area of the gland and overproduction of pro-opiomelanocortin-derived hormones including a-melanocyte stimulating hormone (a-MSH), b-endorphin, adrenocorticotropic hormone (ACTH) and corticotrophin-like intermediate peptide (CLIP). Eventually, the area undergoes micro or macro-adenomatous change (Figure 1).
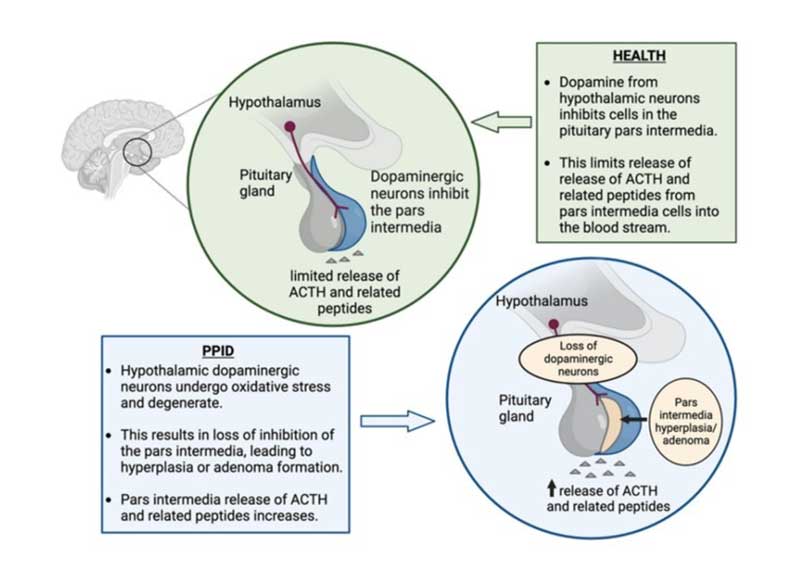
While increased ACTH production occurs, the ACTH secreted is largely biologically inactive and does not result in adrenal stimulation and hypercortisolaemia.
Diagnosis of PPID is based on the signalment, clinical signs and further diagnostic test results. The interpretation of diagnostic tests is influenced by the pre-test probability (the probability of the suspected disease in an individual given their clinical signs).
Positive test results are likely to be inaccurate in animals with a low pre-test probability of PPID, whereas test accuracy is far higher when applied to those considered highly likely to have PPID. Therefore, considering the initial likelihood of PPID prior to diagnostic testing is a vital initial step when making a diagnosis1.
Factors that affect the pre-test probability include age and the specific clinical signs seen in an individual animal.
The condition is seen in older animals; the average age in retrospective case series ranges from 18 to 23 years2-5. In one survey, 21% of horses aged older than 15 years had endocrine changes consistent with PPID6 and in a second, clinical signs consistent with PPID were documented in nearly 40% of horses older than 30 years7.
However, it should be noted that the disease is rarely recognised in animals younger than 10 years, with the youngest ever reported case being in a seven-year-old animal8. Therefore, the age of the animal, and therefore the likelihood of PPID being a true diagnosis, should be considered before further diagnostic testing is undertaken.
The clinical signs associated with PPID can be roughly divided into those that are seen early in the disease and those that are associated with advanced disease (Figure 2). Early signs include loss of topline muscle, change in attitude/lethargy, delayed haircoat shedding, regional hypertrichosis, increased sweating and decreased performance. Advanced signs include generalised hypertrichosis, altered mentation, increased sweating, polyuria/polydipsia and recurrent infections.
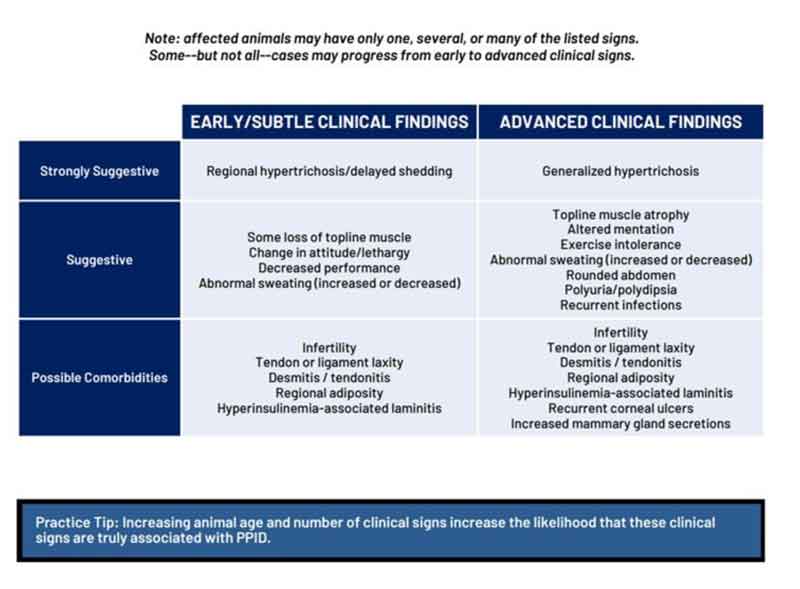
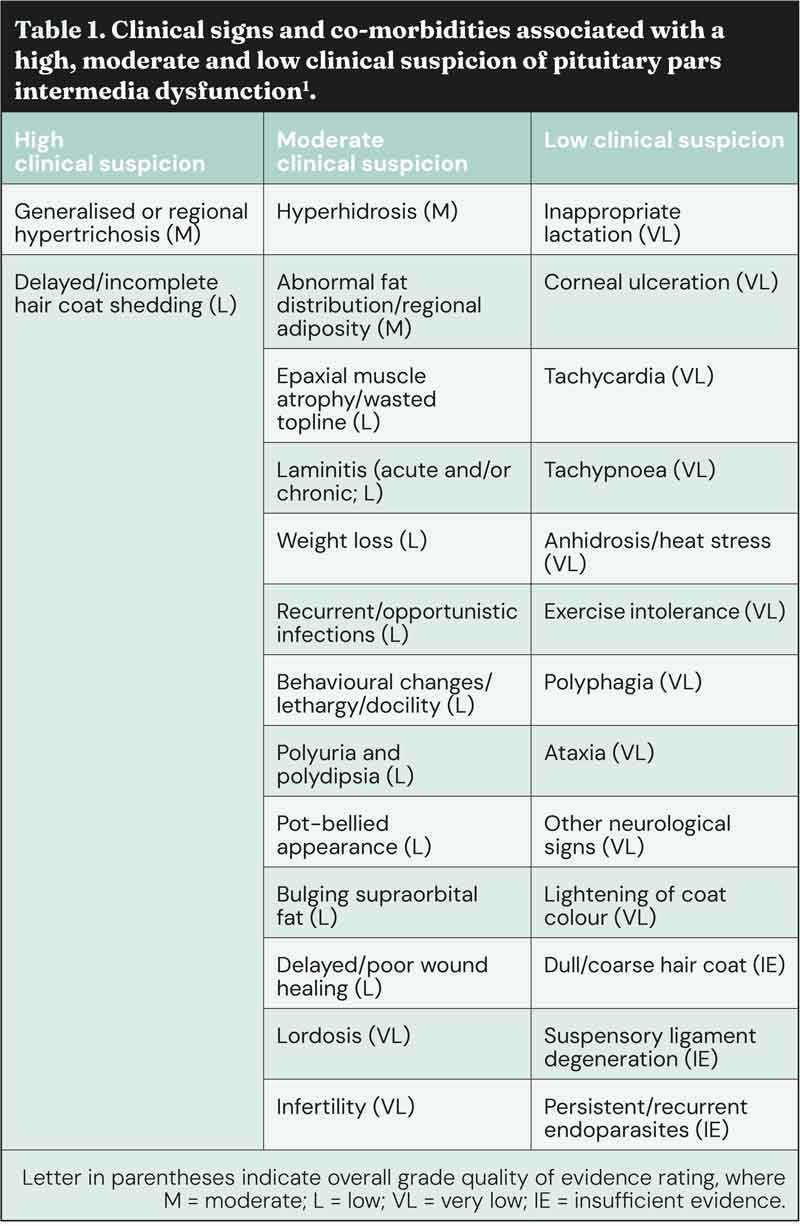
Additionally, they can be divided into those that are highly suggestive of the disease (for example, haircoat changes), those that are moderately suggestive of the disease (for example, loss of topline muscle), and those associated with a low clinical suspicion of the disease (Table 1)1.
Some of the clinical signs associated with PPID are often mistaken by owners as changes associated with ageing. Therefore, owners recognising these signs may not regard them as important enough to seek veterinary advice and animals with PPID may go undetected6. However, it has also been shown that owner are better at detecting hypertrichosis compared to a veterinary clinical examination6. Thus, the historical information provided by the owner is of importance, as well as a detailed clinical examination, to detect the clinical signs associated with PPID.
Hypertrichosis (regional or generalised) is pathognomic for PPID. It occurs due to an increased number of hair follicles found in the anagen, or growth, phase of the hair cycle. The exact cause of this increase is unknown, with theories including pressure on the hypothalamic thermoregulatory centre by the enlarged pituitary or increased pituitary a-MSH production.
Epaxial muscle wastage is common in horses with PPID, and older horses with PPID appear more likely to demonstrate muscle wastage compared to younger horses. The muscle wastage is due to atrophy of types 2A and 2B muscle fibres, but it is unclear whether the atrophy is due to decreased protein synthesis or increased proteolysis.
Weight loss is common in horses with PPID, occurring in up to 88% of animals in published studies. Older horses with PPID appear more likely to suffer from weight loss than younger horses with PPID, but the pathophysiology of the weight loss is unclear. It should be remembered that some horses with PPID will have a normal body condition, and some are even obese; additionally, weight loss should not be confused with epaxial muscle atrophy.
While lethargy is commonly reported in animals with PPID, it also commonly occurs in association with a range of other diseases. Feasibly, increased b-endorphin production by the pars intermedia could be responsible, as b-endorphin is a natural opiate receptor agonist.
Both anhidrosis and hyperhidrosis are reported in animals with PPID, with the former being more common in warmer, humid regions. Again, the exact cause remains unclear.
Hyperhidrosis has been postulated to be a consequence of hypertrichosis, but it persists even after the hair is clipped.
The prevalence of polyuria and polydipsia (PU/PD) in animals with PPID is likely to be under reported due to difficulty monitoring water intake and urine output in pasture-based situations.
The cause of PU/PD is not well understood, but could be the result of reduced anti-diuretic hormone production by the pituitary pars nervosa due to compression by the adjacent enlarged (hypertrophied) pars intermedia.
Hyperhidrosis, fluid loss and resulting increased thirst could also play a role.
Laminitis occurs in around 25% to 50% of animals with PPID, and is associated with insulin dysregulation (ID). The mechanism by which PPID causes ID has yet to be determined; however, it is postulated that some of the pars intermedia derived hormones antagonise insulin (for example, b-cell tropin, which is a breakdown product of CLIP).
Alternatively, in some cases the animal has both equine metabolic syndrome and PPID, and so the ID may have preceded the PPID and just not been recognised. Similarly, the mechanism by which insulin causes laminitis is still unclear.
Theories including glucose deprivation, glucotoxicity, matrix metalloprotease upregulation and increased blood flow-induced hyperthermia have been disproven. Current theories include endothelial dysfunction resulting in vasoconstriction, inappropriate signalling via binding of insulin to insulin-like growth factor receptors, and gut microbiota and gut permeability changes; however, none of these have yet to be definitively proven.
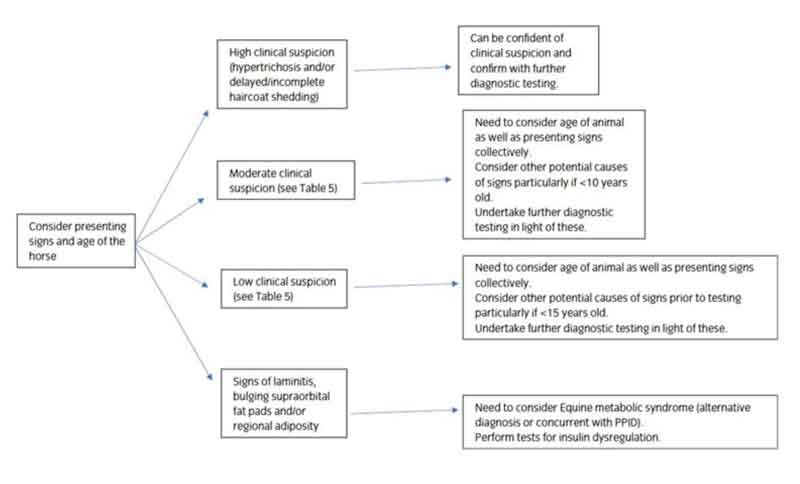
Signs that provide a high index of clinical suspicion and, therefore, the highest pre-test probability for PPID, are current and/or historical hypertrichosis or delayed/incomplete hair coat shedding.
For equids of any age exhibiting clinical signs that provide a moderate index of clinical suspicion for PPID, the combination of signs and age should be considered collectively to inform degree of clinical suspicion before diagnostic testing, and other potential causes of clinical signs should be investigated.
For equids only exhibiting clinical signs that provide a low index of clinical suspicion for PPID, the combination of signs and age should be considered collectively to inform degree of clinical suspicion. Other potential causes of clinical signs should be investigated, and in equids aged less than 15 years this would be advisable before diagnostic testing for PPID, given the low pre-test probability in these animals.
Pre-test probability of PPID is low in equids aged less than 10 years. For equids in this age category, other potential causes of clinical signs should be investigated, and diagnostic testing for PPID should only be considered in the presence of multiple compatible clinical signs that increase clinical suspicion (Figure 3).
No ideal further diagnostic test exists for equine PPID; however, plasma basal ACTH concentrations and the ACTH response to thyrotropin-releasing hormone (TRH) are thought to be the most appropriate tests available (Figure 4 and Table 2).
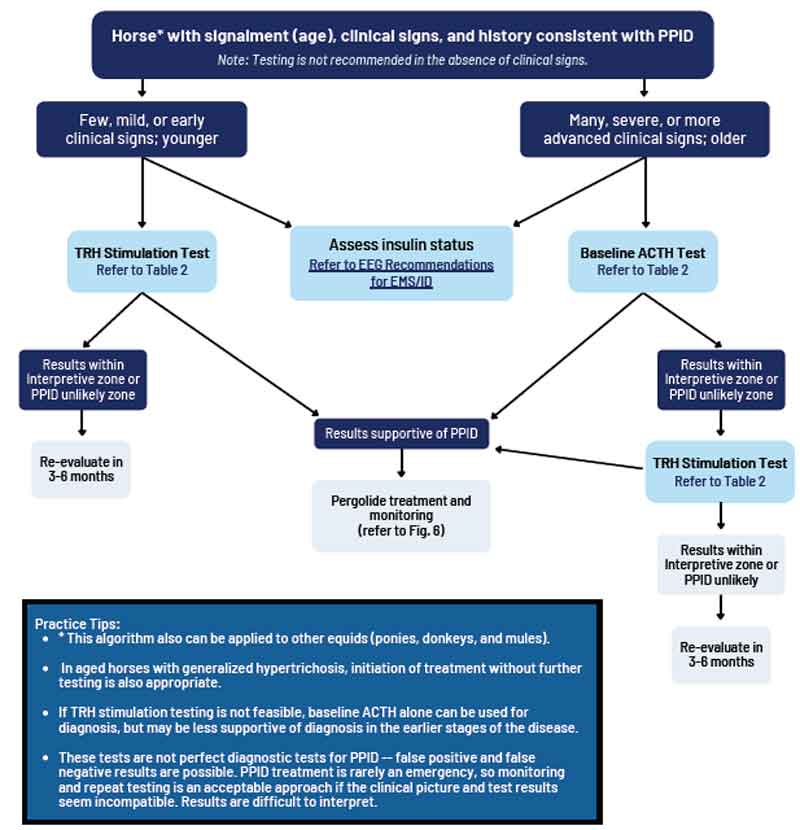
Many factors can influence basal ACTH concentration and it should not be measured immediately after transport, in critically ill animals, or in animals in moderate to severe pain; however, laminitis does not seem to have an adverse impact1.
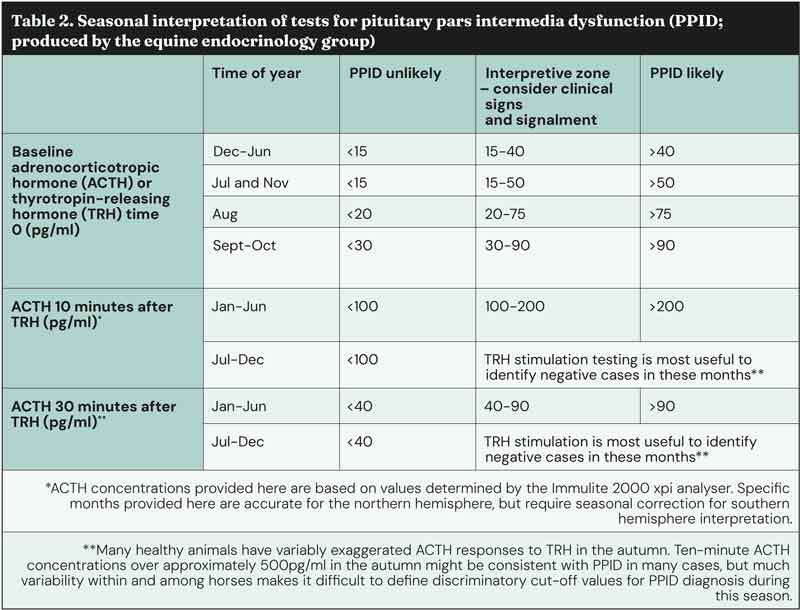
As previously discussed, these results should be interpreted in light of the pre-test probability of PPID in an individual animal and also the season in which the test is being performed. The overall diagnostic accuracy of basal ACTH concentrations for diagnosing PPID in published studies ranged between 88% and 92% in the autumn, and 70% and 86% in the non-autumn, depending on the pre-test probability.
Based on a single published study, the overall diagnostic accuracy of ACTH concentrations in response to TRH after 30 minutes for diagnosing PPID ranged between 92% and 98% in the autumn and 90% and 94% in the non-autumn, depending on the pre-test probability.
The risk of a false positive result increases in situations where a low pre-test probability exists, which could mean that treatment is initiated for PPID without checking for a more likely alternative diagnosis. This could compromise horse welfare due to the commencement of lifelong therapy and/or failing to identify and treat an alternative potentially life-threatening condition.
Additionally, it is recommended that tests for ID are simultaneously performed (Figure 5), as ID occurs in a subset of animals with PPID6,9 and appears to associated with an increased risk of laminitis10, and a worse prognosis11. Appropriate tests for ID include measurement of basal serum insulin concentration, an oral sugar (OST) or glucose test and the insulin tolerance test. While tests for ID and PPID can be performed in the same animal on the same day12, the TRH stimulation test should not be performed immediately after an OST13.
![Figure 5. Algorithm for the detection of insulin dysregulation (reproduced from Equine Endocrinology Group recommendations for the diagnosis and management of equine metabolic syndrome [EMS] 2022; https://equineendocrinologygroup.org).](https://testing-vettimes-next.sfo3.digitaloceanspaces.com/2025/04/Menzies-Gow-Fig-5.jpg)