21 Jul 2020

Laminitis is now considered to be a clinical syndrome associated with systemic disease or altered weightbearing, rather than being a discrete disease entity.
Three forms of laminitis exist – namely sepsis-associated, endocrinopathic and supporting limb. The clinical signs of laminitis remain unchanged; however, it should be remembered it can be subclinical and, as such, will only become evident with the appearance of divergent hoof growth rings.
A new, modified system for laminitis diagnosis and severity scoring has been developed that may aid monitoring of the response to treatment and recovery. Treatment of all types of laminitis includes providing analgesia and foot support, and treating the underlying disease. Digital cryotherapy is also beneficial in cases of sepsis-associated laminitis.
Prevention of all types of laminitis requires prompt treatment of the primary condition and the use of prophylactic digital cryotherapy in animals at risk of sepsis‑associated laminitis.
Laminitis is now considered to be a clinical syndrome associated with systemic disease (sepsis or systemic inflammatory response syndrome [SIRS] or endocrine disease), or altered weightbearing, rather than being a discrete disease entity1.
Three forms of laminitis exist – sepsis-associated, endocrinopathic and supporting limb laminitis (SLL).
Sepsis-associated laminitis occurs secondary to SIRS and/or sepsis, so occurs in animals with severe gastrointestinal disease, pleuropneumonia and septic metritis following retention of foetal membranes.
The carbohydrate overload and black walnut experimental models have been used to investigate the pathogenesis of this form of laminitis2,3; however, the exact identity of the laminitis trigger(s) remains elusive.
These models have produced evidence of systemic inflammation, endothelial activation, leukocyte adhesion and emigration, altered cytokine expression and oxidative injury.
The overall result is failure of critical laminar basal epithelial cell functions and consequent failure of the epithelial adhesion molecules (hemidesmosomes) that attach the epidermal cells to the basement membrane. Laminar separation follows.
Endocrinopathic laminitis is the most common form of laminitis, accounting for 90% of cases of laminitis in some studies4,5.
It includes laminitis associated with insulin dysregulation (ID), as occurs in equine metabolic syndrome (EMS) and a subset of animals with pituitary pars intermedia dysfunction (PPID).
The key feature of EMS is ID, which may manifest in three ways: as hyperinsulinaemia, an excessive insulin response to oral carbohydrate consumption, or tissue insulin resistance6. Additional features include obesity and adipose tissue dysregulation.
PPID is a progressive neurodegenerative disorder associated with loss of the inhibitory dopaminergic input to the pituitary pars intermedia (PPI)7. This results in increased production of the normal hormone products of the PPI – some of which are thought to antagonise the actions of insulin, resulting in ID in a subset of animals.
Lamellar inflammation was not a major histological feature10-12 and evidence of systemic or gastrointestinal inflammation was not apparent. Instead, the lamellar histological changes were more consistent with stretching of the basement membrane, accompanied by increased mitotic activity and cellular proliferation10-12.
Various theories have been postulated to explain the potential relationship between hyperinsulinaemia/ID and laminitis13. The current most popular theory that has some supportive evidence is that, while at physiological concentrations insulin preferentially binds to the insulin receptor, at high concentrations insulin can bind and activate the insulin-like growth factor-1 receptor (IGF-1R)14. Therefore, hyperinsulinaemia may result in overstimulation of IGF-1R-mediated cell proliferation, which, in turn, could potentially weaken the lamellar suspensory apparatus triggering the onset of clinical signs15.
A significant proportion of endocrinopathic laminitis cases occur while the animal is at pasture, and it is postulated that consumption of pasture carbohydrate may exacerbate hyperinsulinaemia – resulting in laminitis.
SLL is uncommon16; however, it is a major contributor to treatment failure in painful limb conditions such as fractures and refractory cases of synovial sepsis.
Little research has been carried out on the pathophysiology of SLL, but studies have suggested cyclic loading of the feet plays an essential role in digital homeostasis at rest, and that decreased frequency of unloading of a limb – combined with increased mean load bearing on that limb – can result in lamellar ischaemia19.
Therefore, SLL may be a consequence of lamellar ischaemia.
A diagnosis of laminitis – regardless of type – is most commonly made based on the clinical signs, which include:

The lameness can vary in severity from that which is only perceptible at the trot, through to spending prolonged periods recumbent.
Traditionally, the Obel method has been used to grade severity (Table 1)20.
| Table 1. The Obel method for grading laminitis severity | |
|---|---|
| Obel grade | >Description |
| Normal | Horse appears sound. |
| 1 | At rest, the horse shifts its weight between the forelimbs; the horse is sound at the walk, but the gait is stilted at the trot in a straight line and on turning. |
| 2 | The gait is stilted at the walk and the horse turns with great difficulty, but one forelimb can be lifted. |
| 3 | The horse is reluctant to walk and one forelimb can only be lifted with great difficulty. |
| 4 | The horse will only move if forced to. |
A new modified laminitis grading system was designed for use in a research setting21 and has now been validated for use in the clinical setting22. It is a 12-point scale based on weight shifting, foot lifting, gait at the walk, gait on a circle and the digital pulse (Table 2), and it may prove useful for monitoring response to treatment and tracking recovery.
| Table 2. The new modified method of laminitis diagnosis and severity scoring | ||||
|---|---|---|---|---|
| Order of examination | Criteria | Description | Points | Points given |
| Stage 1 | ||||
| Examine horse standing | Weight shifting | No weight shifting | 0 | |
| Weight shifting | 2 | |||
| Gently lift each foot up and put back down straight away | Forelimb lift | Prompt and willingly maintained (each forelimb) | 0 | |
| Reluctant and maintained with difficulty (each forelimb) | 1 | |||
| Unable to lift foot/resists attempts to lift foot (each forelimb) | 2 | |||
| Stage 2 | ||||
| Conduct on hard surface. Walk horse approximately 30 minutes | Gait at walk | Normal gait | 0 | |
| Mild short, stilted gait – still moves willingly | 1 | |||
| Moderate short, stilted gait – reluctant/difficult to walk | 2 | |||
| Severe difficulty walking or unable to walk | 6 | |||
| Turn on a short lead in both directions | Gait at circle | Normal circling | 0 | |
| Mild head rise, difficulty when turning, still moves willingly | 1 | |||
| Moderate, sharp head rise, reluctance/difficulty turning | 2 | |||
| Severe difficulty turning, slow and clearly painful | 3 | |||
| Stage 3 | ||||
| All feet must be square in the ground if able to stand | Forelimb digital pulse | Normal | 0 | |
| Bounding (each forelimb) | 2 | |||
| Total score | ||||
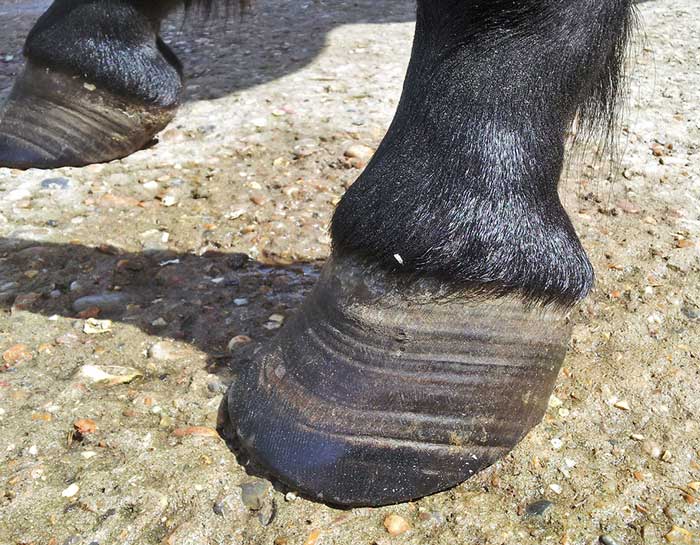
It should be remembered that some episodes of laminitis are subclinical and, while no overt lameness exists, it subsequently becomes apparent as divergent hoof growth rings (Figure 2)1.
Treatment involves providing analgesia and foot support, as well as treating the underlying endocrinopathy or primary condition causing sepsis‑associated laminitis or SLL. Additionally, cryotherapy is indicated in certain circumstances.
NSAIDs are the first choice for analgesia and no evidence exists to suggest any one specific NSAID is superior to the next23.
If NSAIDs do not provide sufficient pain relief then opiates – for example, butorphanol, pethidine and morphine – can be used in addition.
If single drugs do not provide adequate analgesia, multimodal therapy can be used in the hospital setting. Possible combinations typically involve NSAID administration in combination with a constant-rate infusion of lidocaine, ketamine, butorphanol, α-2 agonists or combinations thereof.
A neuropathic component to the pain associated with laminitis has been demonstrated28, making ketamine and gabapentin potentially suitable drugs.
In one study, the combination of tramadol and ketamine resulted in decreased forelimb offloading frequency and increased forelimb loading in horses with naturally occurring laminitis29. Gabapentin improved hindlimb pain that was probably associated with femoral neuropathy in one horse30 and had no apparent adverse effects following oral administration in horses31.
Newer therapies – such as soluble epoxide hydrolase inhibitors and vanilloid receptor antagonists – may prove useful in the future, but, again, further work is needed32.
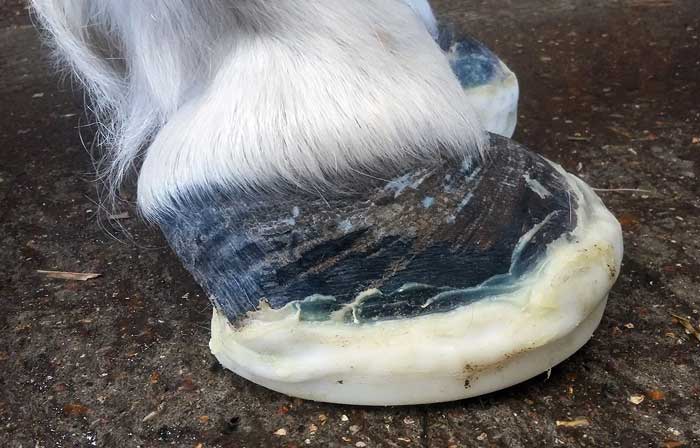
Supporting the foot is essential. Additional support should be applied to the caudal two-thirds of the foot to provide pain relief, and minimise the mechanical forces on the laminae and, hence, pedal bone movement.
The simplest method is to increase the depth of the bedding, ensuring the bedding extends to the door where the horse will spend most of its day. Alternatively or additionally, extra support can be applied directly to the foot itself (Figure 3) using methods that can be broadly divided into frog-only supports, and combined frog and sole supports.
No evidence exists to suggest any one foot support method is superior23.
Vasodilator therapy was historically used based on laminitis being a consequence of digital hypoperfusion, but this pathogenesis concept is outdated for two forms of the condition.
Nevertheless, the sedative effect of acepromazine may have the additional beneficial effect of reducing movement or even resulting in increased periods of time spent recumbent with the weight taken off the feet.
In one study, a trend existed towards use of acepromazine being associated with survival in animals with endocrinopathic laminitis23.
Evidence exists to support the use of cryotherapy in the treatment of sepsis‑associated laminitis.
Hoof temperature should be maintained at lower than 10°C for 72 hours, which can be achieved by immersing the foot and pastern region in ice and water35.
No published evidence exists relating to the use of cryotherapy for treatment of endocrinopathic or SLL.
Animals with acute endocrinopathic laminitis should be removed from pasture and box rested.
A diet based on grass hay (or hay substitute) with low (lower than 10%) non‑structural carbohydrate (NSC) content should be fed and cereals avoided.
Ideally, the forage should be analysed before it is fed. Some recommend soaking hay in water for 30 to 60 minutes before feeding to leach water‑soluble carbohydrates and, therefore, circumvent the need for analysis; however, this does not reliably decrease the NSC content to lower than 10% in all cases36.
Forage-only diets do not provide adequate protein, minerals or vitamins; therefore, a low‑calorie commercial ration balancer product that contains high-quality protein, and a mixture of vitamins and minerals is recommended.
Additional therapies are indicated if an underlying endocrinopathy is confirmed.
The first-choice treatment for PPID is the dopamine agonist pergolide, which replaces the lost dopaminergic inhibition to the PPI and, therefore, reduces hormone production. It is licensed for the treatment of PPID in the horse in the UK and the initial dose is 2µg/kg orally once daily for four to six weeks.
Treatment of EMS should focus on management changes aimed at weight reduction if the animal has regional or generalised adiposity, and exercise, which additionally improves ID.
Weight reduction is achieved through feeding a diet high in fibre and low in NSC. Grain and other concentrated sources of calories should be removed from the diet. Hay or hay substitute should initially be provided at 1.5% of current bodyweight per day, with subsequent further reductions in feed amount depending on the extent of weight loss.
The food should be divided into several smaller feeds per day and strategies used to prolong feed intake time – for example, use of multiple hay nets with small holes.
The optimal amount of exercise required has yet to be determined, but daily exercise once the laminitis has resolved is probably best.
If management changes are unsuccessful, pharmacological interventions involving metformin or levothyroxine can be additionally used in the short term (three to six months).
Prevention of sepsis-associated laminitis involves early and effective treatment of the cause of the sepsis or SIRS, the use of appropriate anti-endotoxic therapy, and the prophylactic use of digital cryotherapy.
Prevention of endocrinopathic laminitis centres on appropriately treating the underlying endocrinopathy, maintaining an optimum body condition and limiting intake of pasture NSC that may exacerbate ID.
A diet based on grass hay (or hay substitute) with low (lower than 10%) NSC content should be fed and cereals avoided. The NSC content of pasture fluctuates widely; therefore, zero grazing might be necessary.
If an animal is to be turned out, steps should be taken to minimise NSC intake – such as the use of a grazing muzzle, strip grazing, rotation of paddocks to keep them at the ideal height or limiting the time spent at pasture.
Forage-only diets do not provide adequate protein, minerals, or vitamins, so a low‑calorie commercial ration balancer product – that contains high-quality protein, and a mixture of vitamins and minerals – is recommended.
If weight gain is required, or the animal is undertaking a large amount of exercise, then caloric intake can be increased by adding unmolassed soaked sugar beet pulp to the diet (0.2kg/day to 0.7kg/day), or by feeding vegetable oil (100ml to 225ml once daily or twice daily, up to a maximum of 100ml/100kg of bodyweight).
Several supplements containing magnesium, chromium or cinnamon – and a variety of herbs – are marketed with claims for improved insulin sensitivity, but scientific evidence of their efficacy in horses is lacking37.
Exercise is also essential in the prevention of laminitis as it has been shown to improve insulin sensitivity and decrease food intake. Light exercise is sufficient to improve insulin sensitivity, but this probably needs to be maintained on a daily basis for the improvement to persist.
Finally, digital cryotherapy reduced the histological severity of laminitis in the hyperinsulinaemia model of laminitis when initiated at the same time as the insulin infusion. However, the clinical applicability for the prevention of endocrinopathic laminitis requires further investigation.
More research is needed to allow specific recommendations for the prevention of SLL to be made.
Some suggest that as limb cycling is an essential component of the circulation, it would be prudent to institute – whenever practicable – measures to improve foot circulation in horses at risk of SLL via either controlled exercise (walking) or physical therapy18.
However, the ideal frequency and duration remains unknown18 and it should be acknowledged this is not possible in the majority of at-risk animals.
Some drugs mentioned in this article are used under the cascade.