12 Jul 2023
Contagious equine metritis – control via industry-led protocol

Image: © callipso88 / Adobe Stock

Contagious equine metritis (CEM) is a venereal disease caused by the fastidious gram-negative bacterium Taylorella equigenitalis, otherwise known as the contagious equine metritis organism (CEMO).
First identified in Newmarket in 1977, subsequent to investigation of a large number of mares displaying excessive purulent vaginal discharge and infertility post-covering, it became an emergent disease that would affect, and be identified in, Thoroughbred populations worldwide.
However, its potential impact on breeding soundness, trade and economies means it is now one of the most internationally regulated equine diseases (Schulman et al, 2013), with most countries imposing import testing regulations.
Indeed, implementation of the Horserace Betting Levy Board (HBLB) Codes of Practice (CoP) in 1978, and enactment of its status as a notifiable disease in 1987, led to the eradication of CEMO from the UK Thoroughbred breeding population, with similar regulations leading to the same status in other countries.
Nonetheless, it remains endemic in non-Thoroughbred populations throughout the world and, in particular, in mainland Europe, making the risk of pathogen re-entry and sporadic outbreaks an ever-present threat.
Over time, and through changing regulations and industry procedures, the landscape of this disease has changed and led to corresponding questioning over, and alteration of, its control mechanisms.
Background
CEMO is a smegma-associated commensal of the external male genitalia transmitted by direct and indirect venereal contact, often during natural cover or application of artificial reproductive techniques.
In mares, infection can result in a range of clinical signs that vary in severity from subclinical through to associated infertility and unpredictable return to oestrus, to endometritis and uncommonly abortion. In stallions, infection is invariably subclinical, but development of a carrier status, which also occasionally occurs in mares, means these animals act as an ongoing potential source of infection for future outbreaks.
Additionally, transplacental transmission or transfer of infection during birth can lead to production of congenitally contaminated foals, of which colts can act as subclinical carriers of CEMO.
Although infection may be mild, self-limiting and resolve without the need for treatment, this ability to develop a carrier status restricts free international trade of horses, and is what necessitates the pathogen’s ongoing stringent control and regulation.
Principles of disease control
With no vaccine available, control revolves around pre-breeding screening of mares and stallions to identify carrier animals and, therefore, prevent them from breeding and spreading infection until CEMO has been successfully eliminated.
Additionally, swabbing and investigation of mares displaying infertility associated with an irregular return to oestrus and/or vaginal discharge during the breeding season acts as a further point of control, through identification of acutely infected animals (Wood et al, 2005).
The HBLB CoP for CEM (https://codes.hblb.org.uk/index.php/page/19) outlines in detail the pre-breeding swabbing protocol, but in short, this requires clitoral and endometrial swabs to be taken from mares, and the urethra, urethral fossa, penile sheath and pre-ejaculatory fluid of stallions to be sampled. All pre-breeding swabs are routinely tested by either PCR, or aerobic and microaerophilic culture at a suitably qualified laboratory, which in the UK must also be BEVA-approved.
The CoP lays out further stringent sampling requirements to demonstrate freedom from infection following treatment of a confirmed CEM case. Here, mares or stallions confirmed to have a CEMO infection must undergo three consecutive sets of post-treatment swabs, with clearance requiring all samples to return negative results on both PCR and microaerophilic culture.
The CoP additionally outline biosecurity measures to be implemented on breeding premises, while for countries wishing to maintain CEM freedom, pre-import and post-quarantine testing of animals prior to the commencement of breeding is mandated.
Implementation of the CoP has proved highly effective in eradicating CEMO following periodic detection in the UK, and in preventing incursion of disease within the Thoroughbred sector globally. Indeed, following decades of surveillance and regulation internationally, the manifestation of CEM as an emergent highly contagious, sexually transmitted disease affecting Thoroughbreds worldwide has largely disappeared (Schulman et al, 2013).
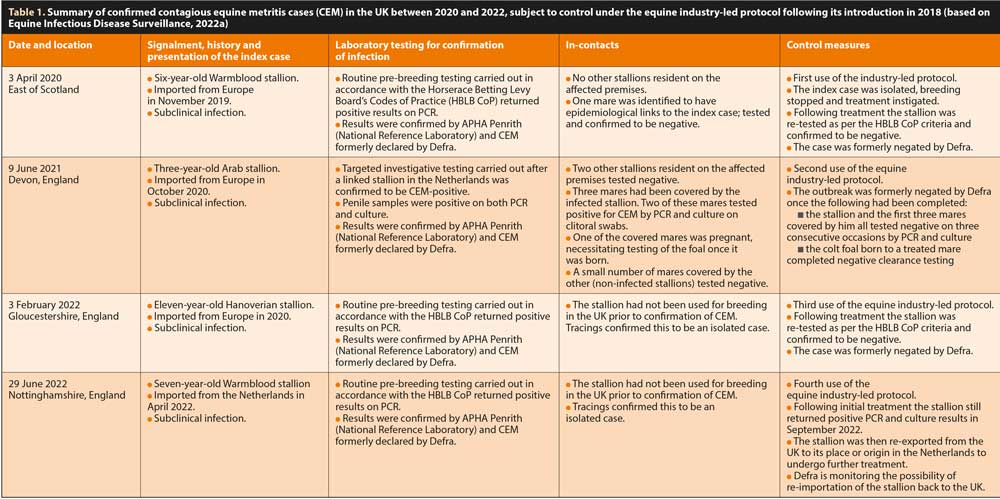
CEMO has not, however, been universally eradicated, but rather the landscape of CEM has changed; most significant outbreaks now demonstrate a different epidemiological manifestation as a disease predominantly associated with artificial reproduction, often in imported non-Thoroughbred horses (Delerue et al, 2019; Schulman et al, 2013). In fact, all the UK’s most recent experiences with CEM (Table 1) have been triggered in non-Thoroughbred stallions imported from mainland Europe.
Lessons learned from previous outbreaks
The epidemiological shift is perhaps best demonstrated by outbreaks recorded in the US (2008-09) and South Africa (2011-12). In these outbreaks, the Thoroughbred population of the affected country remained disease-free, but CEM was shown to have been circulating in the non-Thoroughbred equine population of both countries for some time prior to its identification (Schulman et al, 2013).
In 2008, an American quarter horse stallion in Kentucky underwent routine testing prior to semen export, and was found to be CEMO culture-positive. This triggered an extensive epidemiological tracing programme, which incorporated the testing of approximately 1,000 horses across 48 different states, and which identified a further 22 stallions, one gelding and five mares as positive for CEMO on culture (Erdman et al, 2011).
Investigations indicated that all the positive horses were linked to a single common source, thought to be a Fjord stallion imported into the US in 2000. It was hypothesised that all transmission had likely been via indirect horizontal transfer associated with handling and equipment at large breeding facilities using artificial reproductive techniques (Erdman et al, 2011).
Similarly, the 2011 outbreak in South Africa involved initial identification of four affected horses at an artificial breeding centre, but traceback revealed a further 39 animals to be positive for CEMO on PCR and culture – these were located across 12 further properties in three different provinces (May et al, 2016). These outbreaks demonstrate how artificial reproduction – especially in breeds that do not apply routine pre-breeding screening for CEM – can lead to extensive perpetuation of infection in a country following an initial incursion.
Development of an industry-led protocol to control CEM in the UK
The same epidemiological shift can be seen among UK CEM cases. In 1977, the disease was diagnosed on 29 stud farms in Newmarket, affecting roughly 200 to 250 mares and 23 stallions (Greenwood and Allen, 2020); however, since 1996, it has not been diagnosed in the Thoroughbred population (Wood et al, 2005).
The most recent UK cases have all been primarily associated with detection of CEMO in individual non-Thoroughbred stallions imported from mainland Europe (Equine Infectious Disease Surveillance [EIDS], 2022a; EIDS, 2022b; Meldrum and Newton, 2020). This generally low incursion rate, and the fact that CEMO is a treatable infection, led to a call in 2012 from the UK’s then coalition government to rescind CEM’s notifiable status as part of its “red tape initiative” of removing what was considered outdated legislation.
However, the potential impact of unregulated transmission following disease incursion, as demonstrated by the outbreaks seen in the US and South Africa, remained palpable. This, combined with potential impact on free trade and export markets, led to strong opposition from the equine industry.
After discussions, it was agreed CEM would retain its notifiable status, but on the understanding the industry would take on some of the responsibility for its control.
This led to development of an industry-led CEM control protocol for England, Scotland and Wales, which was introduced as a pilot scheme on 1 February 2018 and, following review, became an established protocol in 2021.
Under the industry-led protocol, overall responsibility for control of CEM as a notifiable disease remains with the Government, but allows industry to take over a share of the managerial, logistical and advice burden. Importantly, the whole protocol is predicated on compliance with the HBLB CoP.
As with any notifiable disease, a non-negative test result or suspicion of CEM should be reported to the APHA, which will then take and test official samples to confirm or negate the presence of CEMO. Confirmation of CEMO on official samples will lead to the relevant UK CVO declaring the presence of a CEM outbreak.
However, under the new protocol, on initial suspicion of CEMO, the owner of the affected animal is given the option by APHA, if they are compliant with the CoP, of following the industry-led control and clearance protocol as opposed to the usual oversight and restrictions imposed by APHA.
If the owner decides to follow the industry-led route, a specialist advisor to the equine industry (EIDS) contacts them within 24 hours (extended to 48 hours for weekends or public holidays) to obtain pertinent information about the affected horse and premises. The specialist industry advisor will then appoint, from an approved list maintained by BEVA, a suitably experienced veterinary surgeon that is registered with Defra as an OV.
To be eligible for inclusion on the BEVA list of approved OVs, a vet must:
- be a current OV, usually with regard to equine export
- have recent and relevant stud farm experience to include sampling the reproductive tract of mares and stallions
- have appropriate knowledge of the HBLB CoP, including demonstrating evidence of issuing pre-breeding screening certificates
It should be noted that the premises’ own veterinary surgeon can be eligible to perform the OV role under the CEM industry-led protocol, but may decline if they consider any conflict of interest is likely to arise.
The appointed OV will initially carry out the official sampling to confirm CEMO, confirm owner and premises compliance with the HBLB CoP, and identify whether any contact tracing is likely to be required – in particular when potentially exposed horses may have moved to other premises.
Once CEM is officially confirmed, the OV will administer treatment, followed by further sampling to demonstrate freedom from disease in accordance with the HBLB CoP. Movement and breeding restrictions are voluntarily self-imposed by the premises themselves, which is in contrast to the mandatory requirements the APHA would normally institute through the serving of statutory notices.
It is worth emphasising that the industry-led protocol relies on voluntary compliance with the CoP and, as a result, will provide owners with a less bureaucratic restriction to the day-to-day running of their lives/business.
If at any time, however, the owner is not fully compliant, then control will revert to the APHA and official restrictions put in place under the Infectious Diseases of Horses Order 1987.
Application of industry-led protocol
Since its introduction in 2018, the industry-led protocol has been used to control four CEM incursions in the UK: one in 2020, one in 2021 and two in 2022 (Table 1).
The protocol is considered to have largely worked effectively in ensuring that, once identified, CEM positive stallions in the UK are restricted from breeding and moving between premises; therefore, preventing any further infectious transmission of CEMO.
What the epidemiology of these four recent CEM outbreaks does highlight, though, is the continuing danger of pathogen incursion through the importation of non-Thoroughbred horses from mainland Europe without specific pre-import testing requirements for CEM.
All four outbreaks involved stallions imported from Europe; infection with CEMO only came to light later when swabbing was carried out prior to commencing a breeding career, or in the 2021 case, following targeted investigative testing. This emphasises the importance and positive impact on the health of the breeding horse population through pre-breeding screening for CEM under the HBLB CoP, but also confirms the continued threat of incursion with the international movement of horses, and potentially also semen.
In conclusion, the landscape of CEM globally has transformed in the past few decades, both in terms of its epidemiology and the nature of disease regulation.
However, what remains constant is the potential for incursion of CEMO into the UK from closely linked neighbouring countries where the pathogen remains endemic.
It is this ever-present threat that necessitates continued regulation and vigilance by the veterinary profession and owners alike.
References
- Delerue M, Breuil M-F, Duquesne F, Bayon-Auboyer MH, Amenna-Bernard N and Petry S (2019). Acute endometritis due to Taylorella equigenitalis transmission by insemination of cryopreserved stallion semen, Journal of Equine Veterinary Science 78: 10-13.
- Equine Infectious Disease Surveillance (2022a). Equine Quarterly Disease Surveillance Report, 18(1), https://www.jdata.co.za/iccviewer/media/dsr20221.pdf
- Equine Infectious Disease Surveillance (2022b). Equine Quarterly Disease Surveillance Report, 18(2), www.jdata.co.za/iccviewer/media/dsr20222.pdf
- Erdman MM, Creekmore LH, Fox PE, Pelzel AM, Porter-Spalding BA, Aalsburg AM, Cox LK, Morningstar-Shaw BR and Crom RL (2011). Diagnostic and epidemiologic analysis of the 2008-2010 investigation of a multi-year outbreak of contagious equine metritis in the United States, Preventative Veterinary Medicine 101(3-4): 219-228.
- Greenwood R and Allen WR (2020). Memories of contagious equine metritis 1977 in Newmarket, Equine Veterinary Journal 52(3): 344-346.
- May CE, Guthrie AJ, Keys B, Joone C, Monyai M and Schulman ML (2016). Polymerase chain reaction-based national surveillance programme to determine the distribution and prevalence of Taylorella equigenitalis in South African horses, Equine Veterinary Journal 48(3): 307-311.
- Meldrum KC and Newton JR (2020). Contagious equine metritis: a 2020 vision on control of a notifiable disease in the United Kingdom, Equine Veterinary Journal 52(3): 347-348.
- Schulman ML, May CE, Keys B and Guthrie AJ (2013). Contagious equine metritis: artificial reproduction changes the epidemiologic paradigm, Veterinary Microbiology 167(1-2): 2-8.
